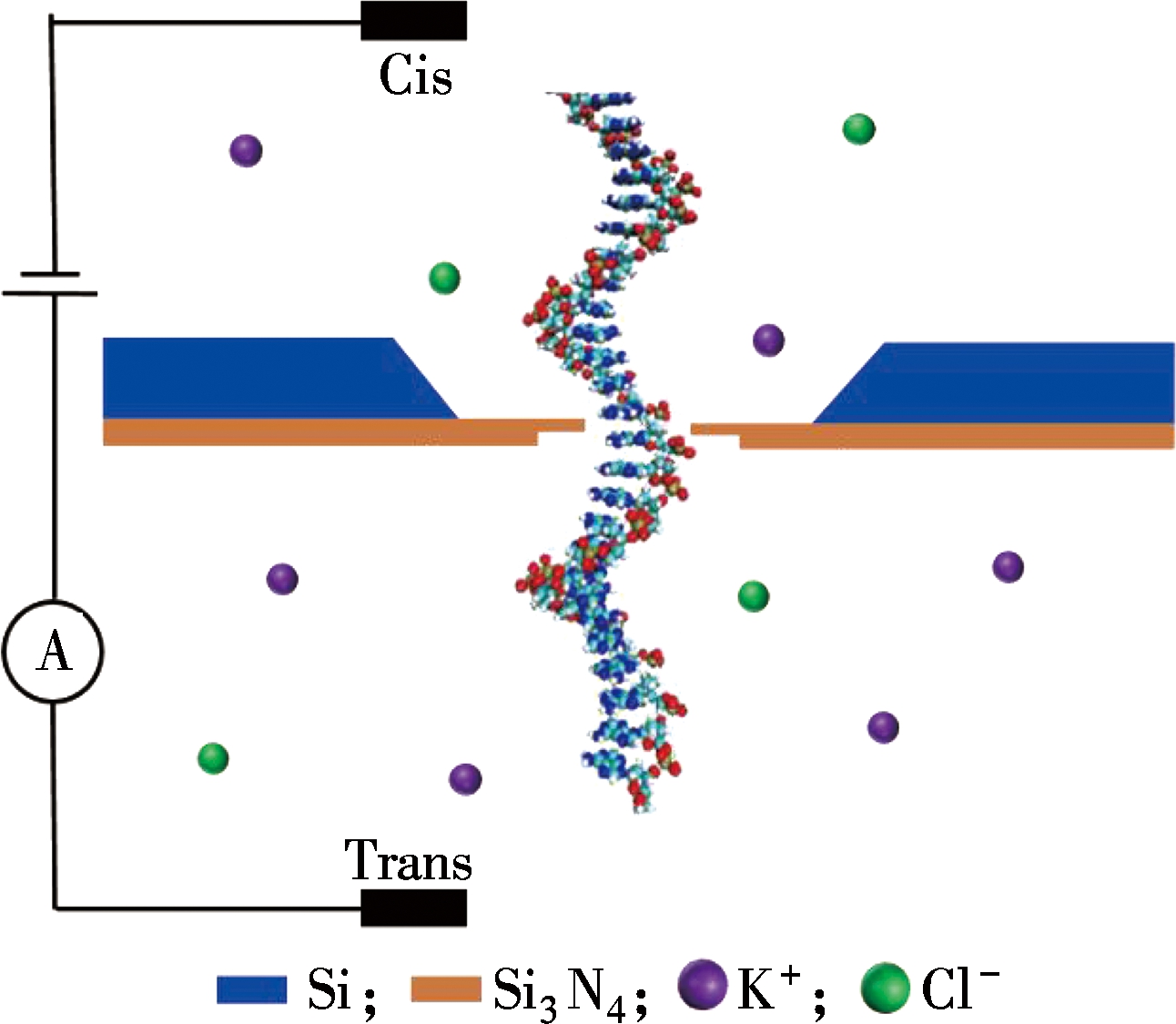
Fig.1 Schematic of the experimental system setup
Abstract:Aiming at the issues of controlling the translocation speed of DNA through a solid-state nanopore and enlarging the signal-to-noise ratio of ionic current modulation, which are challenges for the application of nanopore technology in DNA detection, salt concentration gradients are applied across the nanopore to investigate their influence on the DNA translocation time and signal-to-noise ratio. Experimental data demonstrates that, in symmetric concentration conditions, both the current blockade and dwell time forλ-DNA translocation through a solid-state nanopore increase along with potassium chloride concentration. When the concentration in the trans chamber is decreased from 1 to 0.1 mol/L, keeping the concentration of the cis chamber at 1 mol/L, the normalized current blockade is found to be increased by one order. The increased dwell time and enhanced signal-to-noise ratio are achieved with salt gradients across the nanopore, which can improve the sensitivity when detecting DNA samples.
Key words:solid-state nanopore; salt gradients;λ-DNA; signal-to-noise ratio; current blockade; dwell time
In biological systems, it is a common phenomenon that polymers can electrophoretically translocate through nanometer-sized apertures in cell membranes. In 1996, Kasianowicz et al.[1] first demonstrated that an applied electric potential could drive charged polymers to translocate through the nanopores and induce a modulated electrical conductance of the nanopore as the polymers were transiting the nanopore. This has the potential to be used to characterize the polymers, such as DNA, RNA, and proteins[2-4]. During the past decade, a number of experimental studies[2-5] on the electrophoretic translocation of polymers across nanopores were conducted. It is believed that nanopore technology has the most potential for achieving low-cost and high-throughput DNA sequencing. Solid-state nanopores have emerged as a versatile alternative to biological nanopores because of their well-defined geometries, stability, ease of integration and compatibility with various electronic or optical measurement techniques[6-8]. Over the last few years, solid-state nanopore technology[9-11] has been widely used for the detection and differentiation of DNA nucleotides. Although solid-state nanopores have obvious advantages over biological nanopores (such as MspA and α-hemolysin nanopores), the noise level of ionic current in solid-state nanopores (about 10 to 100 pA) is about 10 times greater than that of biological nanopores[12-13]. Therefore, elevating the signal-to-noise ratio is a priority for realizing nanopore DNA sequencing. Moreover, DNA transits so fast through the nanopore that the current signal cannot contain adequate information of a single base. Then, controlling the translocation speed of a DNA strand to guarantee temporal and spatial resolution still remains a considerable challenge. In this paper, the effects of salt concentration gradients across the Si3N4 nanopore on DNA translocation time and the signal-to-noise ratio are investigated. It is found that the DNA translocation process can be significantly affected by changing the salt concentration of the electrolyte in both the trans and cis chambers. In addition, it is also found that the blockade current increases with enforced salt concentration gradient, which is beneficial for increasing the signal-to-noise ratio. It is believed that the experimental results provide insights for the design of DNA sequencing devices.
As shown in Fig.1, a 100-nm-thick Si3N4 membrane supported on a silicon chip is fabricated by lithography and wet etching processes. The Si3N4 membrane was milled to reduce the film thickness from 100 nm to around 10 nm by focused ion beam (FIB). After the milling process, a transmission electron microscope (TEM) was used to fabricate nanopores. All nanopore chips were subjected to a standard clean process in piranha solution, which is a mixture of H2SO4 and H2O2 (volume ratio 3∶1), for 15 min and treated in an oxygen plasma environment for 15 s prior to use. The membrane chip was mounted on a polymethylmethacrylate (PMMA) flow cell. Silicone elastomer gaskets were used to seal the chip, ensuring the nanopore as the only connection between the two chambers filled with potassium chloride (KCl) electrolyte, which was degassed, filtered, and adjusted to pH 8.0 at room temperature. The λ-DNA purchased from Takara BIO Inc. was dispersed into the cis chamber. Both the analyte (cis) and the collection (trans) chambers were equipped with an Ag/AgCl electrode. As an external bias voltage is applied, DNA will be electrophoretically driven though the Si3N4 nanopore and cause a pulse signal accordingly. A resistive feedback amplifier (HEKA EPC10, HEKA Elektronik) was used at 100 kHz with low-pass filtering at 3 kHz. All measurements were conducted inside a dark Faraday cage.

Fig.1 Schematic of the experimental system setup
2.1 Symmetric salt concentrations
Firstly, the symmetric concentrations were used to study the KCl concentration dependency of DNA translocation through a solid-state nanopore. The KCl concentration was set as 0.2, 0.4 and 0.6 mol/L in both the trans and cis chambers, respectively. A bias voltage of 1 000 mV was applied. In all the concentrations investigated, the macroscopic conductivities were almost linear with KCl concentration.
Fig.2 shows the scatter plots of the normalized current blockade IB/I0 (where IB is the current blockade and I0 is the open pore current) versus the translocation time when λ-DNA is driven through a 68 nm nanopore with 0.2, 0.4 and 0.6 mol/L KCl, respectively. The histograms of current blockade distributions and dwell time distributions are also plotted in Figs.3(a) and (c). As demonstrated in the figures, only one peak exists for both the histograms of current blockade and dwell time once the Gaussian distribution function is used to fit these histograms. By analyzing the histograms for the current blockade, it is believed that λ-DNA translocates through the nanopore in a linear conformation[5,11].
As shown in Fig.3(a), there is an obvious peak for the histograms of current blockade in each experiment conducted at a specified KCl concentration. The Gaussian distribution function was used to fit the data based on the least square method. The peak values IB are 320, 483 and 618 pA for the 0.2, 0.4 and 0.6 mol/L KCl concentrations, respectively. It is found that the maximum KCl concentration produces the largest blockade ionic current as shown in Fig. 3(b). The work done by Stein et al.[14] showed that the conductance in nanochannels was approximately linear to the KCl concentration between 0.1 and 1 mol/L. In this range of salt concentration, the ionic current I is directly proportional to the ion concentration n, as a limiting case for the Levine relationship[15], I~n(1+C), where C is the correction to the bulk conductivity. Based on the traditional Ohm’s law[16], both the open pore current and current drop are linear with KCl concentration[17].
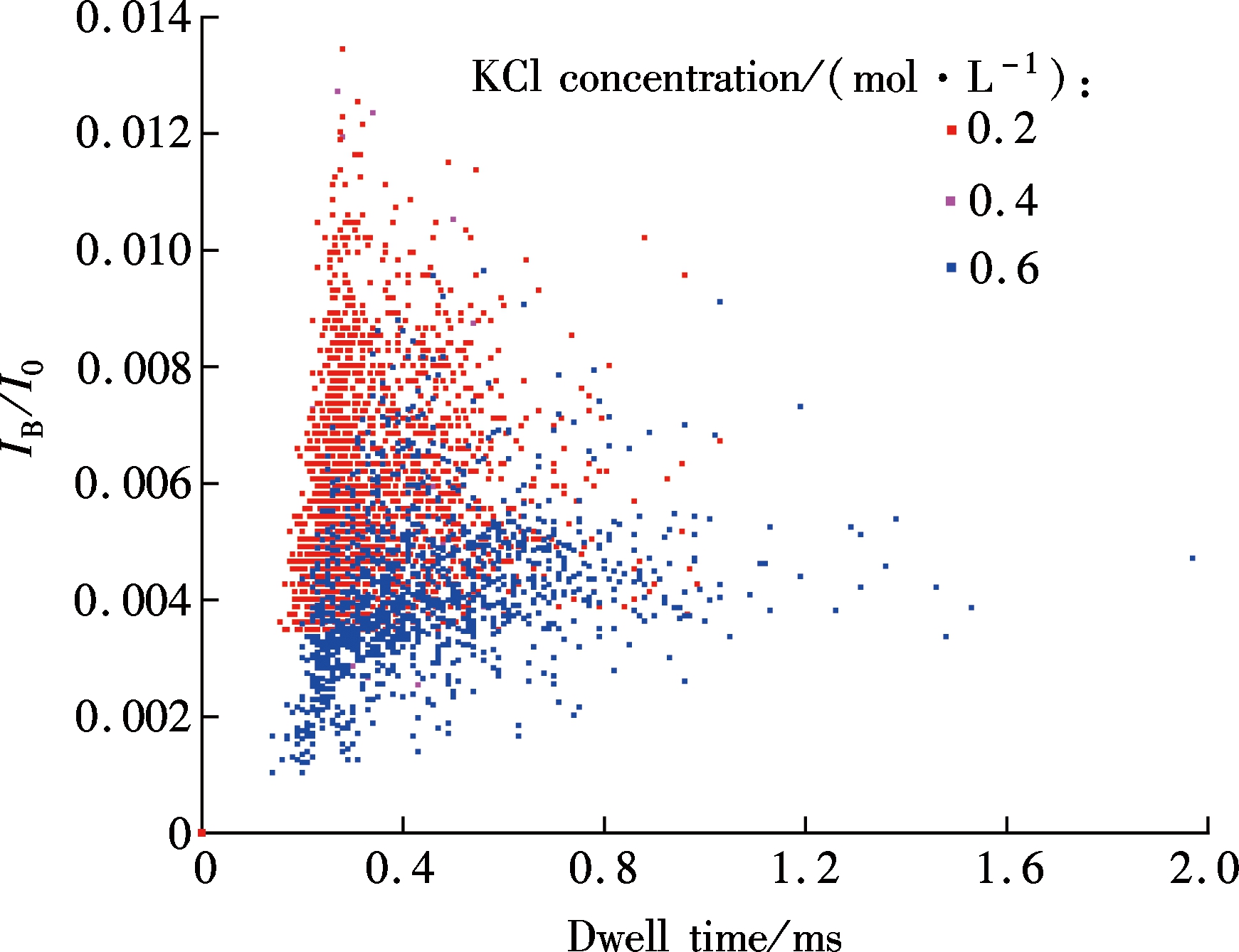
Fig.2 Scatter plots of normalized current blockade versus dwell time
The histograms of translocation time for λ-DNA transport through the nanopore with different concentrations are plotted in Fig.3(b). Similarly, all experimental data was fitted with Gaussian curves using the least square method. The Gaussian peak values are 270, 358 and 370 μs for 0.2, 0.4 and 0.6 mol/L KCl, respectively. Fig.3(d) shows the relationship between the DNA translocation time through the nanopore and KCl concentration. Clearly, the dwell time for λ-DNA translocating through the nanopore increases with the KCl concentration ranging from 0.2 to 0.6 mol/L, as plotted in Fig.3(d).
2.2 Asymmetric salt concentrations
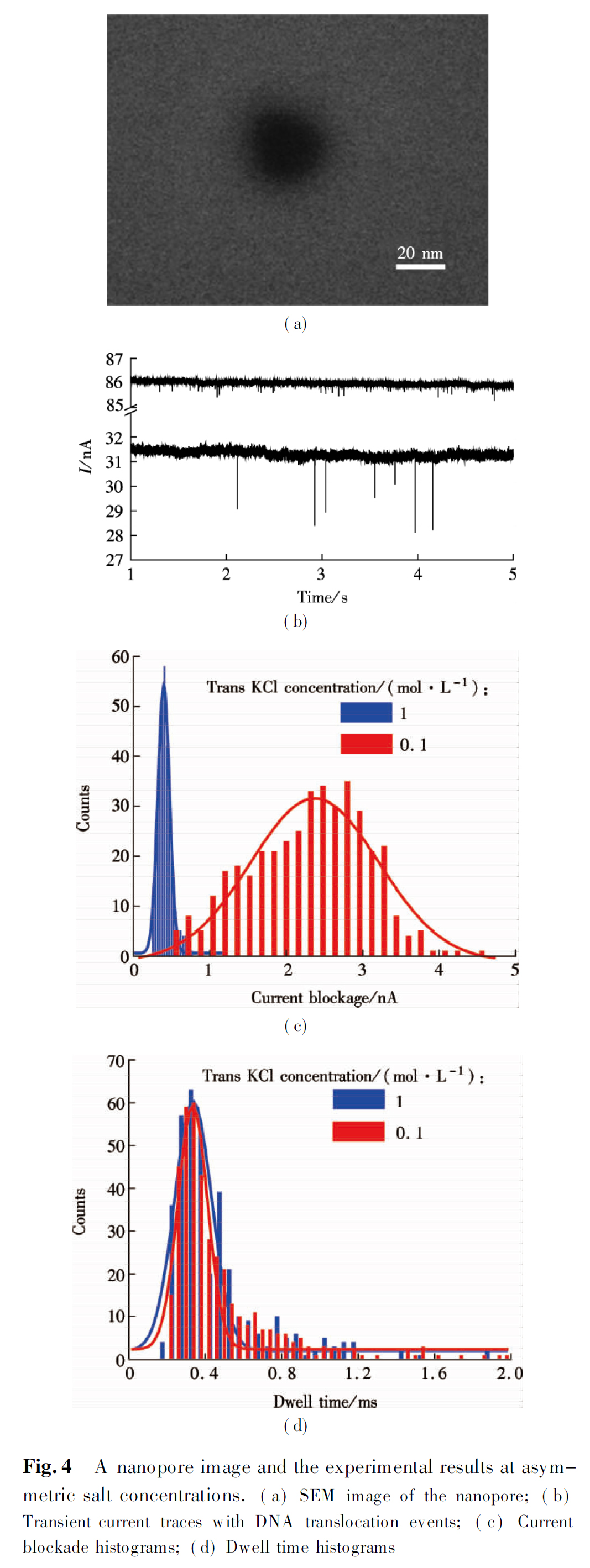
Having investigated the impact of KCl concentration on DNA translocation through solid-state nanopores, we subsequently studied the effect of salt concentration gradients on the DNA transport process. λ-DNA transport events were observed for different trans and cis KCl concentrations at a fixed bias voltage of 500 mv when maintaining a constant concentration of 3 μg/mL λ-DNA in the cis chamber. Ionic current traces measured through a 25 nm nanopore is shown in Fig.4(b) and the histograms of current blockade distributions are plotted in Fig.4(c) for two different salt concentrations (namely, 0.1 and 1 mol/L KCl) in the cis chamber. As shown in Fig.4(c), the Gaussian distribution function is used to fit the data based on the least square method. There is one obvious Gaussian peak for the histogram of blockade current for each KCl concentration gradient. For 1 mol/L KCl in the trans chamber, the peak value IB is 396 pA and the open pore current I0 is 85 nA. For 0.1 mol/L KCl in the trans chamber, the peak value IB is 2 372 pA and the open pore current I0 is 31 nA. Interestingly, when the concentration in the trans chamber was decreased from 1 to 0.1 mol/L, the normalized current blockade increased by more than one order.

Simultaneously, we also find that enhanced current blockade with increasing salt gradients is accompanied by no distinct change of DNA translocation time as shown in Fig.4(d). The experimental results can be explained by a combination of three effects. First, under salt concentration gradients, the applied voltage may result in cation selectivity[18]. Diffusion of K+ ions from the cis to trans chamber is hindered by the applied potential. As negative ions are continuously pumped into the trans chamber, the pore vicinity is effectively polarized. A higher voltage drop outside the pore diminishes the electric field inside the pore. Secondly, a decreased electrosomotic flow of K+ counterions along the DNA and pore surface provides less drag force opposing DNA motion in the pore[19-20]. Therefore, balanced by two contrary effects, DNA translocation time did not change notably.
The above experiments clearly demonstrate that the DNA translocation process can be affected by changing the salt concentration of electrolyte in the trans and cis chambers. In symmetric concentration environments, both the open pore current and current drop increase linearly with KCl concentration. The dwell time for λ-DNA translocating through a nanopore increases as the KCl concentration increases. In addition, imposed salt concentration gradient across the pore causes an apparent current blockade increase, with no change of the DNA translocation time. When the salt concentration of the trans chamber was decreased from 1 to 0.1 mol/L, the normalized current blockade (IB/I0) increased by at least one order.
It means that the modulation of salt concentration can help researchers decrease the transport speed of DNA and a salt concentration gradient will also enhance the signal-to-noise ratio vastly. We hope the results in this paper can provide some helpful suggestions for the development of nanopore technology in DNA sequencing.
References:
[1]Kasianowicz J J, Brandin E, Branton D, et al. Characterization of individual polynucleotide molecules using a membrane channel[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(24): 13770-13773. DOI:10.1073/pnas.93.24.13770.
[2]Bezrukov S M, Vodyanoy I, Parsegian V A. Counting polymers moving through a single ion channel[J]. Nature, 1994, 370(6487): 279-281. DOI:10.1038/370279a0.
[3]Reiner J E, Balijepalli A, Robertson J W F, et al. Disease detection and management via single nanopore-based sensors[J]. Chemical Reviews, 2012, 112(12): 6431-6451. DOI:10.1021/cr300381m.
[4]Miles B N, Ivanov A P, Wilson K A, et al. Single molecule sensing with solid-state nanopores: Novel materials, methods, and applications[J]. Chemical Society Reviews, 2013, 42(1): 15-28. DOI:10.1039/c2cs35286a.
[5]Fologea D, Uplinger J, Thomas B, et al. Slowing DNA translocation in a solid-state nanopore[J]. Nano Letters, 2005, 5(9): 1734-1737. DOI:10.1021/nl051063o.
[6]Haque F, Li J, Wu H C, et al. Solid-state and biological nanopore for real-time sensing of single chemical and sequencing of DNA[J]. Nano Today, 2013, 8(1): 56-74. DOI:10.1016/j.nantod.2012.12.008.
[7]Schneider G F, Dekker C. DNA sequencing with nanopores[J]. Nature Biotechnology, 2012, 30(4): 326-328. DOI:10.1038/nbt.2181.
[8]Dekker C. Solid-state nanopores[J]. Nature Nanotechnology, 2007, 2(4): 209-215. DOI:10.1038/nnano.2007.27.
[9]Verschueren D V, Jonsson M P, Dekker C. Temperature dependence of DNA translocations through solid-state nanopores[J]. Nanotechnology, 2015, 26(23): 234004. DOI:10.1088/0957-4484/26/23/234004.
[10]Nicoli F, Verschueren D, Klein M, et al. DNA translocations through solid-state plasmonic nanopores[J]. Nano Letters, 2014, 14(12): 6917-6925. DOI:10.1021/nl503034j.
[11]Uplinger J, Thomas B, Rollings R, et al. K+, Na+, and Mg2+ on DNA translocation in silicon nitride nanopores[J]. Electrophoresis, 2012, 33(23): 3448-3457. DOI:10.1002/elps.201200165.
[12]Beamish E, Kwok H, Tabard-Cossa V, et al. Precise control of the size and noise of solid-state nanopores using high electric fields[J]. Nanotechnology, 2012, 23(40): 405301. DOI:10.1088/0957-4484/23/40/405301.
[13]Garaj S, Hubbard W, Reina A, et al. Graphene as a subnanometre trans-electrode membrane[J]. Nature, 2010, 467(7312): 190-193. DOI:10.1038/nature09379.
[14]Stein D, Kruithof M, Dekker C. Surface-charge-governed ion transport in nanofluidic channels[J]. Physical Review Letters, 2004, 93(3): 035901. DOI:10.1103/PhysRevLett.93.035901.
[15]Levine S, Marriott J R, Neale G, et al. Theory of electrokinetic flow in fine cylindrical capillaries at high zeta-potentials[J]. Journal of Colloid and Interface Science, 1975, 52(1): 136-149. DOI:10.1016/0021-9797(75)90310-0.
[16]Kowalczyk S W, Grosberg A Y, Rabin Y, et al. Modeling the conductance and DNA blockade of solid-state nanopores[J]. Nanotechnology, 2011, 22(31): 315101. DOI:10.1088/0957-4484/22/31/315101.
[17]Smeets R M M, Keyser U F, Krapf D, et al. Salt dependence of ion transport and DNA translocation through solid-state nanopores[J]. Nano Letters, 2006, 6(1): 89-95. DOI:10.1021/nl052107w.
[18]Wanunu M, Morrison W, Rabin Y, et al. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient[J]. Nature Nanotechnology, 2010, 5(2): 160-165. DOI:10.1038/nnano.2009.379.
[19]van Dorp S, Keyser U F, Dekker N H, et al. Origin of the electrophoretic force on DNA in solid-state nanopores[J]. Nature Physics, 2009, 5(5): 347-351. DOI:10.1038/nphys1230.
[20]Luan B, Aksimentiev A. Electro-osmotic screening of the DNA charge in a nanopore[J]. Physical Review E, 2008, 78(2): 021912. DOI:10.1103/PhysRevE.78.021912.
Citation:Yu Jingwen, Si Wei, Sha Jingjie, et al. Effect of salt gradients on DNA translocation through solid-state nanopores[J].Journal of Southeast University (English Edition),2016,32(3):307-311.DOI:10.3969/j.issn.1003-7985.2016.03.008.
DOI:10.3969/j.issn.1003-7985.2016.03.008
摘要:针对DNA过孔速度过快以及信噪比低等制约固态纳米孔应用于DNA分子检测的问题,通过改变纳米孔两侧盐溶液的浓度,研究了其对DNA的过孔时间以及信噪比的影响.实验结果表明,当纳米孔两侧氯化钾溶液浓度相同时,λ-DNA的过孔时间以及电流堵塞信号都随着氯化钾浓度的提高而增加.但当纳米孔cis端的KCL浓度保持在1 mol/L,trans端的氯化钾浓度由1 mol/L变为0.1 mol/L时,归一化后的电流信号幅值增加了1个数量级.因此,调整纳米孔两端盐溶液的浓度梯度可以有效降低DNA的过孔速度和提高信噪比,从而大大提高了纳米孔在DNA分子检测方面的灵敏度.
关键词:固态纳米孔;盐浓度梯度;λ-DNA;信噪比;堵塞电流;过孔时间
中图分类号:TH789; Q786
Received:2015-12-28.
Foundation item:s:The National Natural Science Foundation of China (No. 51435003, 51375092), Fundamental Research Funds for the Central Universities, the Innovative Project for Graduate Students of Jiangsu Province (No.KYLX_0100), the Scientific Research Foundation of Graduate School of Southeast University (No.YBJJ1540).
Biographies:Yu Jingwen (1992—), female, graduate; Chen Yunfei (corresponding author), male, doctor, professor, yunfeichen@seu.edu.cn.