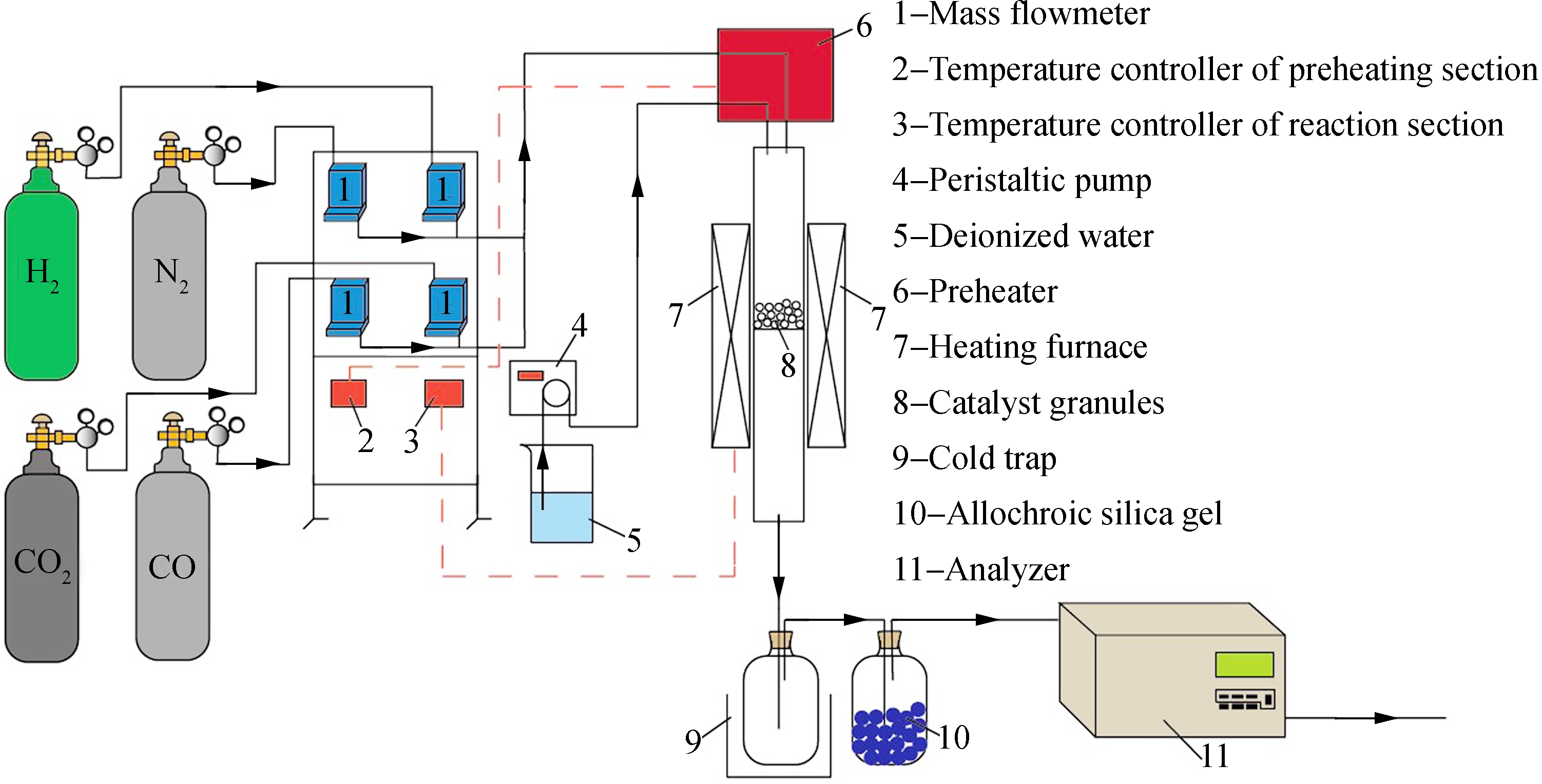
Fig.1 Schematic diagram of biomass gasification fuel gas methanation reaction
Abstract:Ni-based catalysts supported by γ-Al2O3 were prepared for improving the lower heating value (LHV) of biomass gasification fuel gas through methanation. Prior to the performance tests, the physico-chemical properties of the catalyst samples were characterized by N2 isothermal adsorption/desorption, X-ray diffraction (XRD) and a scanning electron microscope (SEM). Afterwards, a series of experiments were carried out to investigate the catalytic performance and the results show that catalysts with 15% and 20% Ni loadings have better methanation catalytic effect than those with 5% and 10% Ni loadings in terms of elevating the LHV of biomass gasification fuel gas. Moreover, controllable influential factors such as the reaction temperature, the H2/CO ratio and the water content occupy an important position in the methanation of biomass gasification fuel gas. 15Ni/γ-Al2O3 and 20Ni/γ-Al2O3 catalysts have a higher CO conversion and CH4 selectivity at 350 ℃ and the LHV of biomass gasification fuel gas can be largely increased by 34.3 % at 350 ℃. Higher H2/CO ratio and a lower water content are more beneficial for improving the LHV of biomass gasification fuel gas when considering the combination of both CO conversion and CH4 selectivity. This is due to the fact that a higher H2/CO ratio and lower water content can increase the extent of the methanation reaction.
Key words:Ni-based catalyst; methanation; biomass gasification fuel gas; lower heating value
It is generally accepted that energy and fossil fuel consumption are crucial to industrial and economic development. However, traditional fossil fuels such as coal and petroleum have a limited storage amount and also can cause various severe environmental problems such as air pollution and the greenhouse effect during use. Biomass fuel gas generated from gasification of organic materials rooting in forestry and agricultural residues, for instance, wood and straw, can be utilized as an alternative, clean and supplementary source of fuel[1]. Hence, production of biomass fuel gas through gasification has attracted more and more attention recently due to the increasing market demand and the encouragement of the national energy policy. Nevertheless, the lower heating value (LHV) of some types of biomass gasification fuel gas is relatively small (2 to 6 MJ/m3 under standard temperature and pressure) due to different biomass sources and gasification conditions[2-6]. From perspective of combustion, a smaller LHV potentially implies a larger amount of fuel gas when the same amount of heat is in need, which accordingly raises the operation costs of a few factories and plants. Furthermore, gas appliances are designed in accordance with certain heating values. Biomass gasification fuel gas with an insufficient heating value might cause undesired damage to them and influence the efficiency of combustion. Therefore, it is economically and securely meaningful to improve the LHV of biomass gasification fuel gas.
Two technologies have been frequently employed to enhance the lower heating value of biomass gasification fuel gas: 1) Decreasing the content of N2 contained in the gas; 2) Converting CO and H2 into CH4 through methanation. The first technology normally includes the process of N2 separation from the product gas, which correspondingly increases the content of toxic CO and explosive H2, thus increasing the complexity and risk to the system. Besides, choosing some novel substances such as steam[7-8] or oxygen-enriched air[9-10] as biomass gasification agents can reduce the dilution effect of N2 on the LHV of biomass gasification fuel gas as well. Similarly, this method may also decrease maneuverability. Methanation of syngas has been investigated extensively over a century. It is generally applied to produce high purity methane from syngas (CO+H2) out of coal gasification or to remove CO from ammonia synthesis feed gas (N2+H2) in ammonia plants[11-12]. In recent years, this technology has played a significant role in the elimination of CO impurities from reformate gas for polymer electrolyte fuel cells (PEFCs) or polymer electrolyte membrane fuel cells (PEMFCs)[13-14]. However, from the current literature, applying the technical approach of methanation into the biomass gasification fuel gas system for LHV enhancement is rarely reported.
According to the research results of Ma et al.[15], the CO disproportionation reaction, also known as Boudouard reaction[16] which leads to carbon deposition, can be inhibited by adding water vapor to the system. Considering the existence of water during our research, the effect of this side reaction can be ignored. The main reaction in our system, which refers to methanation reaction, is highly exothermic. Massive chemical energy will be transformed into thermal energy with the reaction proceeding, which naturally causes a considerable loss of energy, thus leading to the reduction of thermal efficiency. In addition, the volumetric flow rate of the product gas will decrease with the increase in the generation of CH4 since the methanation reaction belongs to volume-reduced reactions. Inadequate product gas volume will have impact on the use continuity of gas appliances. Consequently, sufficient assurance of product gas volume should be taken into consideration for the purpose of industrial application when aiming at increasing the LHV of the biomass gasification fuel gas through methanation. Though the LHV of pure CH4 is higher than that of single-component CO and H2(35.914, 12.636 and 10.785 MJ/m3 for CH4, CO and H2, respectively), the formation of CH4 is based on the CO and H2, which also have a certain amount of heating value that can partially account for the total LHV of the biomass gasification fuel gas. In a nutshell, it is not optimum to carry out methanation as thoroughly as possible from those aspects above.
The key factor of methanation is the catalyst and vast quantities of noble-metal and transition-metal methanation catalysts have been studied[17]. In comparison with metals such as Ru, Rh and Co[18-20], the Ni-based catalyst is more appealing for methanation due to its massively successful industrial application and lower market price. Many efforts have been made to enhance the performance of the Ni-based methanation catalysts in order to produce a higher quality methane or to remove CO from the ammonia synthesis feed gas more thoroughly. The results of Lu et al.[21] indicated that VOx can promote the dissociation of CO in the methanation reaction and thus yield more methane. Kim et al.[13] reported that Ir-doped Ni catalysts showed the best performance for decreasing the CO content from reformate gas below 10×10-6 at 190 to 230 ℃. As for the catalyst support, various metal oxides such as Al2O3, SiO2, ZrO2 and TiO2 can be used as methanation catalyst support. Of all these materials, Al2O3 is the most typical one for methanation. Liu et al.[22] systematically compared three Ni-based catalysts and concluded that the low-temperature activity of the three catalysts are in the order of Ni/Al2O3>Ni/ZrO2>Ni/CeO2. Unlike the systems mentioned above, our goal in this work is to improve the heating value of biomass gasification fuel gas by partial methanation. As a result, there is no need to remove CO as utterly as possible by selective methanation or to produce high quality methane by complete methanation on the premise of the expected requirements. Accordingly, the catalysts employed in the biomass gasification fuel gas system will act differently from those catalysts described above.
In this paper, with the purpose of studying the feasibility and practicality of biomass gasification fuel gas LHV improvement through methanation, a systematic inquiring experiment was carried out. In terms of the catalysts for methanation, we selected nickel as the active metal and γ-Al2O3 as the support due to its abundance and various advantages such as high stability and having a satisfactory porous structure. In order to examine whether Ni was successfully loaded on the support and observe the surface difference of the catalysts with diverse Ni loadings, N2 isothermal adsorption/desorption, X-ray powder diffraction (XRD) and a scanning electron microscopy (SEM) were used to characterize the catalyst samples.
The Ni/γ-Al2O3 catalysts with different Ni loadings (5%, 10%, 15% and 20%) were prepared by an impregnation method and the whole preparation process was elucidated as follows. First, the γ-Al2O3 granular support (2 to 3 mm) was impregnated with an aqueous solution of Ni(NO3)2 ·6H2O. During the period of impregnation, the mixture was kept at 60 ℃ for 12 h so as to obtain catalyst precursor samples with uniformly dispersed Ni species. Secondly, after impregnation, all samples were dried at 120 ℃ overnight and subsequently calcined at 450 ℃ for 4 h in air. In order to describe the catalysts with different Ni loadings conveniently, the abbreviations of the catalysts are denoted as θNi/γ-Al2O3(θ=5, 10, 15 and 20) in this paper.
The X-ray diffraction (XRD) analysis of the catalyst samples were measured on an X-ray diffractometer (Smart Lab, Japan) with Cu Kα radiation under a 0.02° scanning step and a 20 (°)/min scanning speed operating at 40 kV and 100 mA. The wavelength λ was 0.154 nm and the scanning 2θ angle range was from 20° to 80°. The phase identification was carried out using the professional processing software Jade.
The specific surface area of the support and catalysts was evaluated by the BET method using N2 adsorption at -196 ℃ via an automated gas adsorption analyzer (BELSORP-Mini, Japan). All the samples were pretreated under N2 flow at 150 ℃ for 1 h prior to N2 adsorption. The morphology of catalyst samples was determined by a HITACHI S-4800 scanning electron microscopy (SEM) operating at 5 kV.
The biomass gasification fuel gas methanation tests were conducted in a continuous flow fixed-bed reactor equipped with a stainless steel tube (inner diameter: 50 mm) under atmospheric pressure (see Fig.1). The cylinder gas was employed to simulate the composition of the biomass gasification fuel gas and the feed gas flow was controlled by the mass flowmeter. A peristaltic pump was employed to guide the water to the reactor tube. A preheater under 250 ℃ was utilized to preheat the feed gas and transform the liquid water into steam. The reaction temperature was measured in the middle of the cata-
lyst bed by a K-type thermocouple. In a typical methanation reaction, 50 mL catalysts were reduced in a gas flow of H2(GHSV: 1 000 h-1) at 450 ℃ for 2 h. When the oven was cooled down to 200 ℃, the reduction gas was subsequently switched to the reaction feed gas(GHSV: 2 000 h-1). All the tests were performed in a temperature range of 300 to 500 ℃. The composition of dry gaseous products was analyzed by VARIO PLUS (MRU, Germany) posterior to a water trap and the gas analyzer is accurate to the 0.01% level.

Fig.1 Schematic diagram of biomass gasification fuel gas methanation reaction
The process of biomass gasification fuel gas methanation possibly includes the following main reactions:
CO+3H2![]() CH4+H2 ΔH0=-206.1 kJ/mol
CH4+H2 ΔH0=-206.1 kJ/mol
(1)
CO2+4H2![]() CH4+2H2O ΔH0=-165.0 kJ/mol
CH4+2H2O ΔH0=-165.0 kJ/mol
(2)
CO+H2O![]() CO2+H2 ΔH0=-40.6 kJ/mol
CO2+H2 ΔH0=-40.6 kJ/mol
(3)
2CO![]() CO2+C ΔH0=-173.0 kJ/mol
CO2+C ΔH0=-173.0 kJ/mol
(4)
In light of the reactions above, the LHV of the biomass gasification fuel gas (unit: MJ/m3) is calculated as
LHV=10.785VH2+12.636VCO+35.914VCH4
(5)
The percentage conversion of CO (XCO) and CH4 product selectivity (SCH4) are determined by[23]
XCO=100×([FCO]in-[FCO]out)/[FCO]in
(6)
SCH4=![]()
(7)
where V is the volume fraction and F is the molar flow rate of the inlet and outlet species.
2.1 Textural properties and phase compositions of the catalysts
Tab.1 exhibits the textural properties of the support and prepared catalysts. The γ-Al2O3 support is provided with a high surface area and pore volume. The addition of Ni can significantly decrease the BET surface area and pore volume. However, the shrunken surface area of the catalysts are still considerable enough for catalytic performance which can be proven in our following experiments. In addition, the pore volume and the average pore diameter descend with Ni loading. The pore size distribution and N2 adsorption/desorption isotherms of the support and catalysts are shown in Fig.2. It can be seen that the γ-Al2O3 support exhibits a pore size distribution centered at 3.82 nm. The addition of Ni to the support shifts the pore size distribution to smaller sizes. Most of the pore size concentrates on the range of 2 to 10 nm. Judging from the patterns of isotherms in Fig.2(b), the γ-Al2O3 support and catalysts are with mesoporous structure (2 to 50 nm) according to the IUPAC classifications for the isotherms[24].
As shown in Fig.3, in comparison with the material PDF cards, the diffraction peaks of four different Ni-load-ing catalysts appeared at 37.3°, 43.3°, 62.9° and 75.5° are assigned to distinct peaks of the NiO (PDF#75-0197). The XRD patterns of 5Ni/γ-Al2O3 and 10Ni/γ-Al2O3 catalysts do not show peaks at 62.9° and 75.5° due to the high dispersion of the NiO species. In addition, the unique peaks observed at 45.7° and 67.3° correspond to distinct peaks of γ-Al2O3 (PDF#04-0880).These indicate that Ni was successfully loaded on the γ-Al2O3 carrier. Besides, Fig.3 displays some discrepancy among four catalysts. It can be seen that the intensity of NiO diffraction peaks increases and half peak bandwidth decreases with the increase in Ni loading, which suggests that the grain size of NiO becomes larger when more Ni is loaded on the γ-Al2O3 support[25]. This can be deduced by the Debye-Scherrer formula (D=Kγ/Bcosθ) and explained by the fact that overlarge Ni loadings will reduce the degree of NiO dispersion, thus easily leading to the aggregation of Ni species on the support.
Tab.1 Textural properties of the support and catalysts
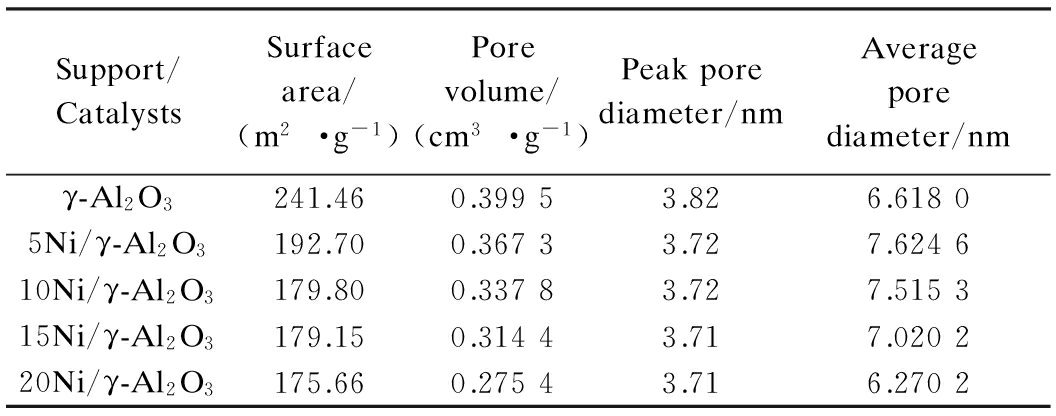
Support/CatalystsSurfacearea/(m2·g-1)Porevolume/(cm3·g-1)Peakporediameter/nmAverageporediameter/nmγ-Al2O3241.460.39953.826.61805Ni/γ-Al2O3192.700.36733.727.624610Ni/γ-Al2O3179.800.33783.727.515315Ni/γ-Al2O3179.150.31443.717.020220Ni/γ-Al2O3175.660.27543.716.2702
(a)

(b)
Fig.2 N2 isothermal adsorption/desorption analysis. (a) Pore size distribution; (b) N2 adsorption/desorption isotherms

Fig.3 XRD patterns of Ni/γ-Al2O3 catalyst samples
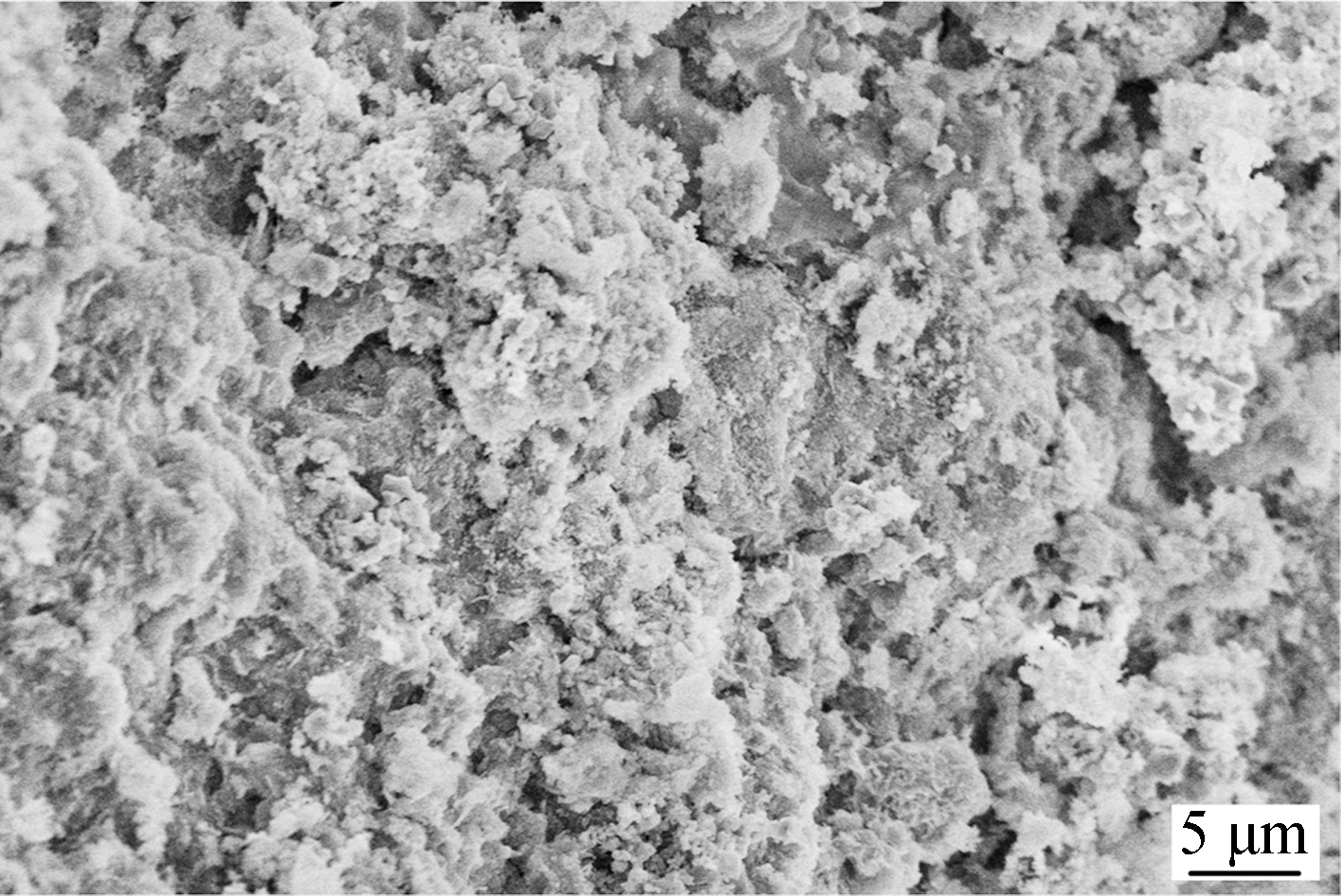
SEM images in Fig.4 display the texture properties of four catalysts with different Ni loadings. It can been seen

(a)

(b)
(c)

(d)
Fig.4 SEM images of γ-Al2O3 supported Ni catalysts. (a) 5Ni/γ-Al2O3; (b) 10Ni/γ-Al2O3; (c) 15Ni/γ-Al2O3; (d) 20Ni/γ-Al2O3
that catalysts with less Ni loading (5Ni/γ-Al2O3, 10Ni/γ-Al2O3) show uneven structure while Ni species on the catalysts with more Ni loading (15Ni/γ-Al2O3, 20Ni/γ-Al2O3) disperse uniformly. Notably, a NiO cluster can be clearly observed in the SEM image of 20Ni/γ-Al2O3, which is consistent with the XRD results.
As shown in Fig.5, the LHV of biomass gasification fuel gas can be increased to a various extent through methanation over Ni/γ-Al2O3 catalysts (The dotted line in Fig.5 represents the original LHV of biomass gasification fuel gas before methanation). All the catalysts with different Ni loadings presented catalytic performance and revealed the same changing pattern. For one certain catalyst, the LHV increases with the increase in reaction temperature to a peak then decreases. As for 5% and 10% Ni loading catalysts, the LHV reaches maximum at 400 ℃ while for the 15% and 20% Ni loading ones, the crest value of LHV appears at 350 ℃.

Fig.5 LHV of biomass gasification fuel gas after methanation reaction with the dry feed gas consisting of 46.4% H2, 18.6% CO, 27.0% CO2, 8.0 % N2and the liquid water flow rate of 7.63 mL/h
The relationship between CO conversion and reaction temperature is displayed in Fig.6. Notably, the line plots for all the catalysts reveal the same pattern. The CO conversion reaches a maximum value at 350 ℃. Compared to other catalysts, 20Ni/γ-Al2O3 has the highest CO conversion and 5Ni/γ-Al2O3 has the lowest CO conversion. Tab.2 lists the methane selectivity of the catalysts applied in the tests. As can be seen, the 15% and 20% Ni loading catalysts have the higher methane selectivity at 350 ℃ in contrast with other reaction temperatures. This might explain the fact that the LHV of biomass gasification fuel gas after methanation reaches a maximum value at 350 ℃ when combined with CO conversion, as shown in Fig.6. However, in terms of 5% and 10% Ni loading catalysts, the methane selectivity at 400 ℃ is higher than those at other temperatures though the CO conversion at 400 ℃ is not the highest. Therefore, the LHV attains a crest value at 400 ℃.
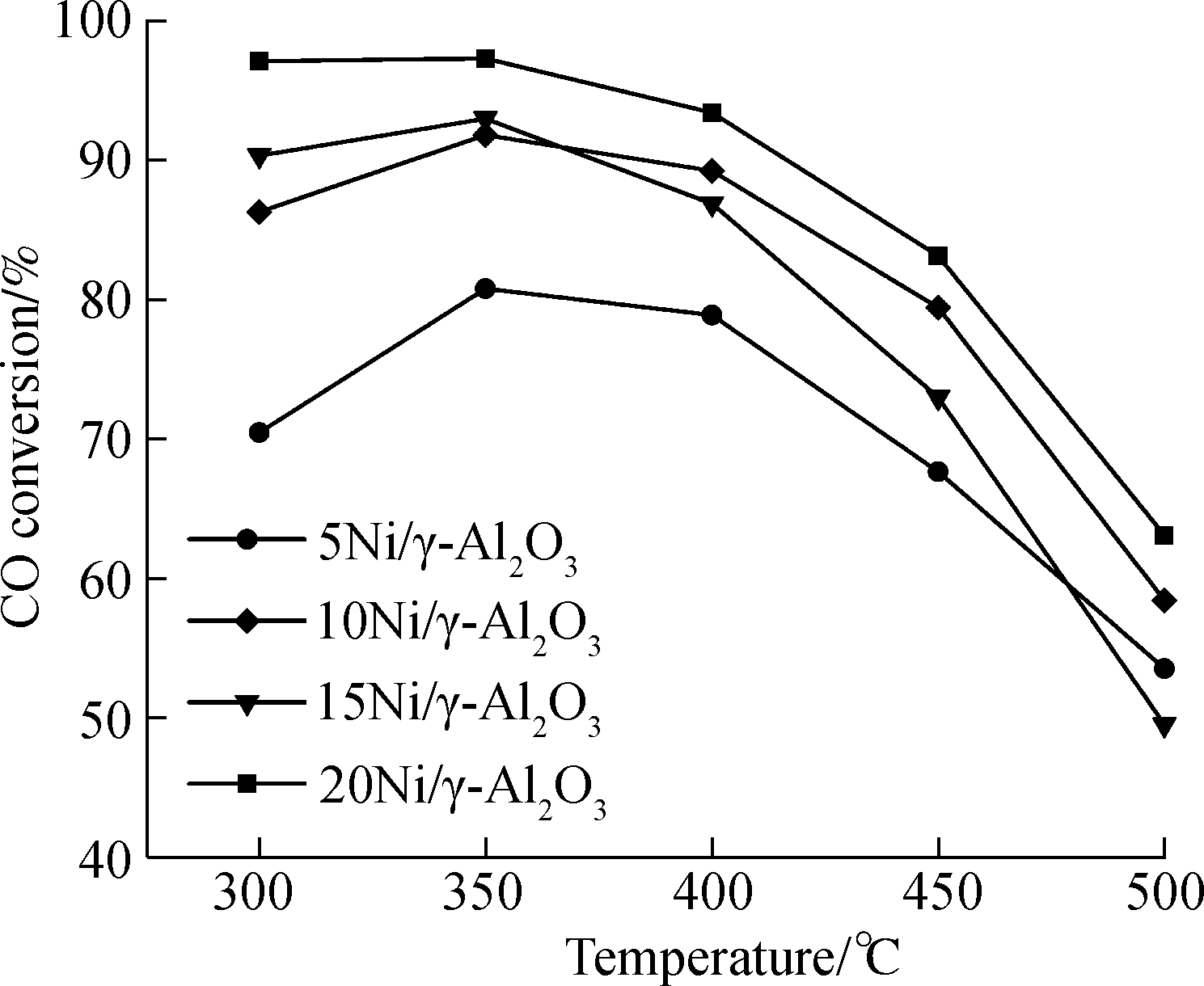
Fig.6 CO conversion with the dry feed gas consisting of 46.4% H2, 18.6% CO, 27.0% CO2, 8.0% N2and the liquid water flow rate of 7.63 mL/h
Tab.2 Methane selectivity of catalysts with different Ni loadings under test temperatures
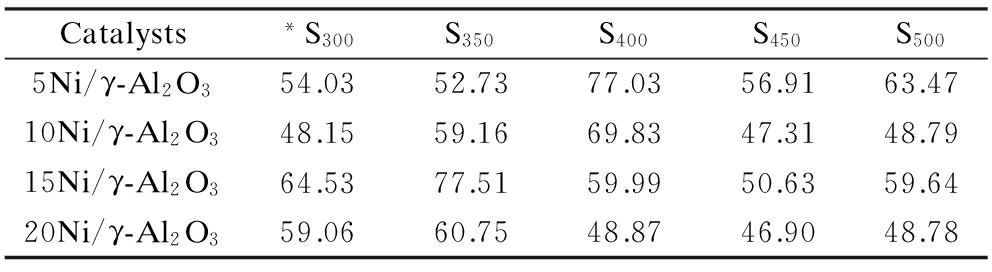
Catalysts*S300S350S400S450S5005Ni/γ-Al2O354.0352.7377.0356.9163.4710Ni/γ-Al2O348.1559.1669.8347.3148.7915Ni/γ-Al2O364.5377.5159.9950.6359.6420Ni/γ-Al2O359.0660.7548.8746.9048.78
Note:*represents the methane selectivity under 300 ℃ and the rest follow in the same manner.
As shown in Fig.7, the LHV growth rate of biomass gasification fuel gas after methanation increases with the increase in the H2/CO ratio. Best of all, the LHV of biomass gasification fuel gas can be improved by 34.3% when the H2/CO ratio is 2.5 for 15Ni/γ-Al2O3 catalyst. According to Eq.(1), the stoichiometric ratio of H2/CO is 3, and from the perspective of reaction kinetics, more H2 will promote the reaction. Therefore, the LHV has the largest growth rate when the H2/CO ratio is 2.5 under the selected experimental conditions. Moreover, it can be seen that the LHV of biomass gasification fuel gas is difficult to enhance, it even decreases when the H2/CO ratio is 0.8 or 1.0. This is due to the fact that under those circumstances, the H2/CO ratio is far lower than the stoichiometric ratio of the methanation reaction. In consequence, the degree of the reaction is small and CH4 production is insignificant. Hopefully, referring to the process of synthetic natural gas production from coal, adding a technology of water gas shift reaction (WGS) before methanation[11] or developing bifunctional catalysts combined with WGS and methanation[15] may be able to ease such a situation. For the 20Ni/γ-Al2O3 catalyst, it is noteworthy that the LHV growth rate at the H2/CO ratio of 2.5 is smaller than other catalysts. This phenomenon may be explained by the fact that powerfully released reaction heat will intensify the aggregation of the Ni species and/or cause sintering, thus reducing the catalytic effect[26]. According to SEM images in Fig.4, 20Ni/γ-Al2O3 catalyst presents a NiO cluster.
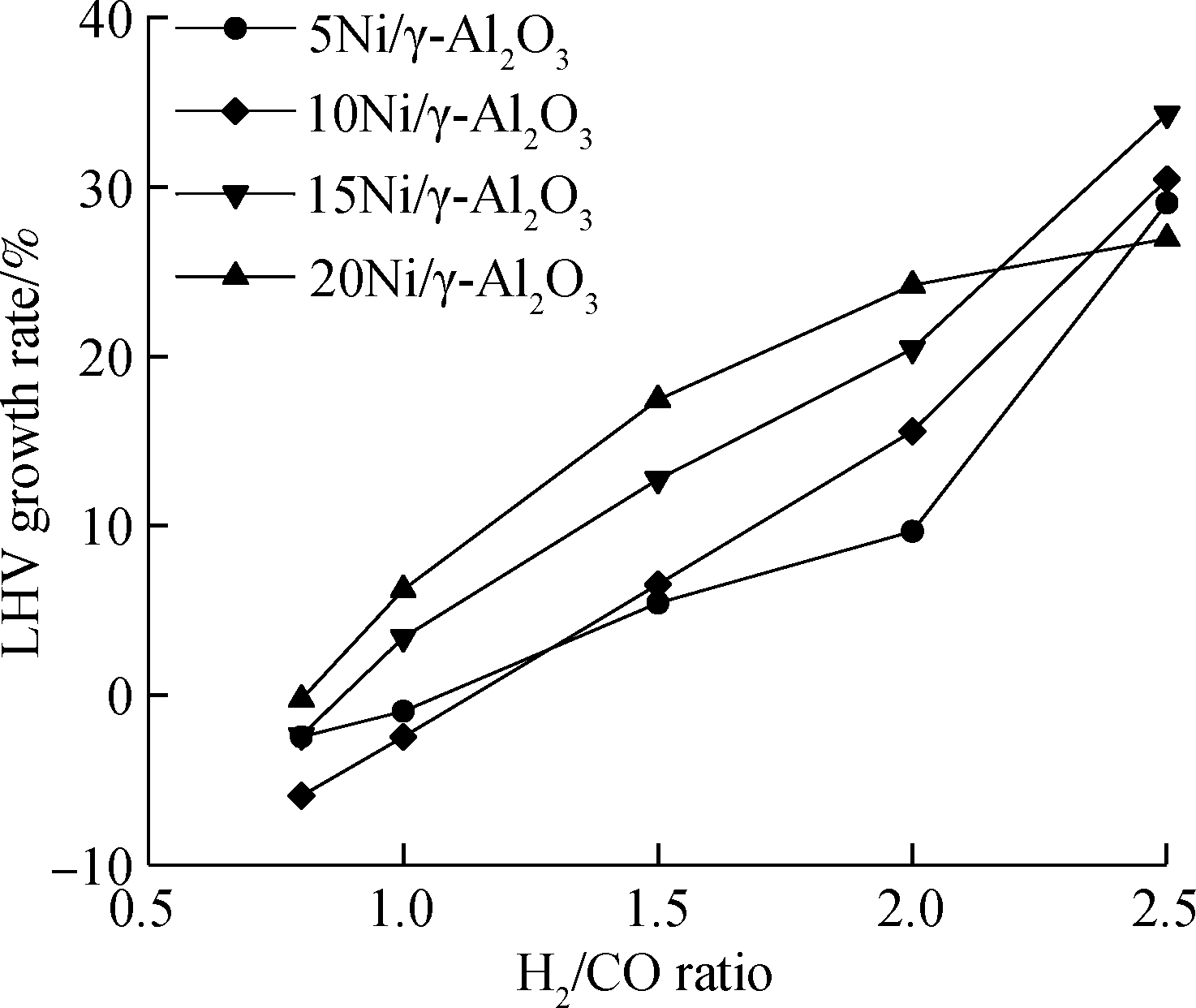
Fig.7 LHV growth rate of biomass gasification fuel gas after methanation at 350 ℃ with the dry feed gas consisting of 27.0% CO2, 8.0% N2, the H2/CO ratio varying from 0.8 to 2.5 and the liquid water flow rate of 7.63 mL/h
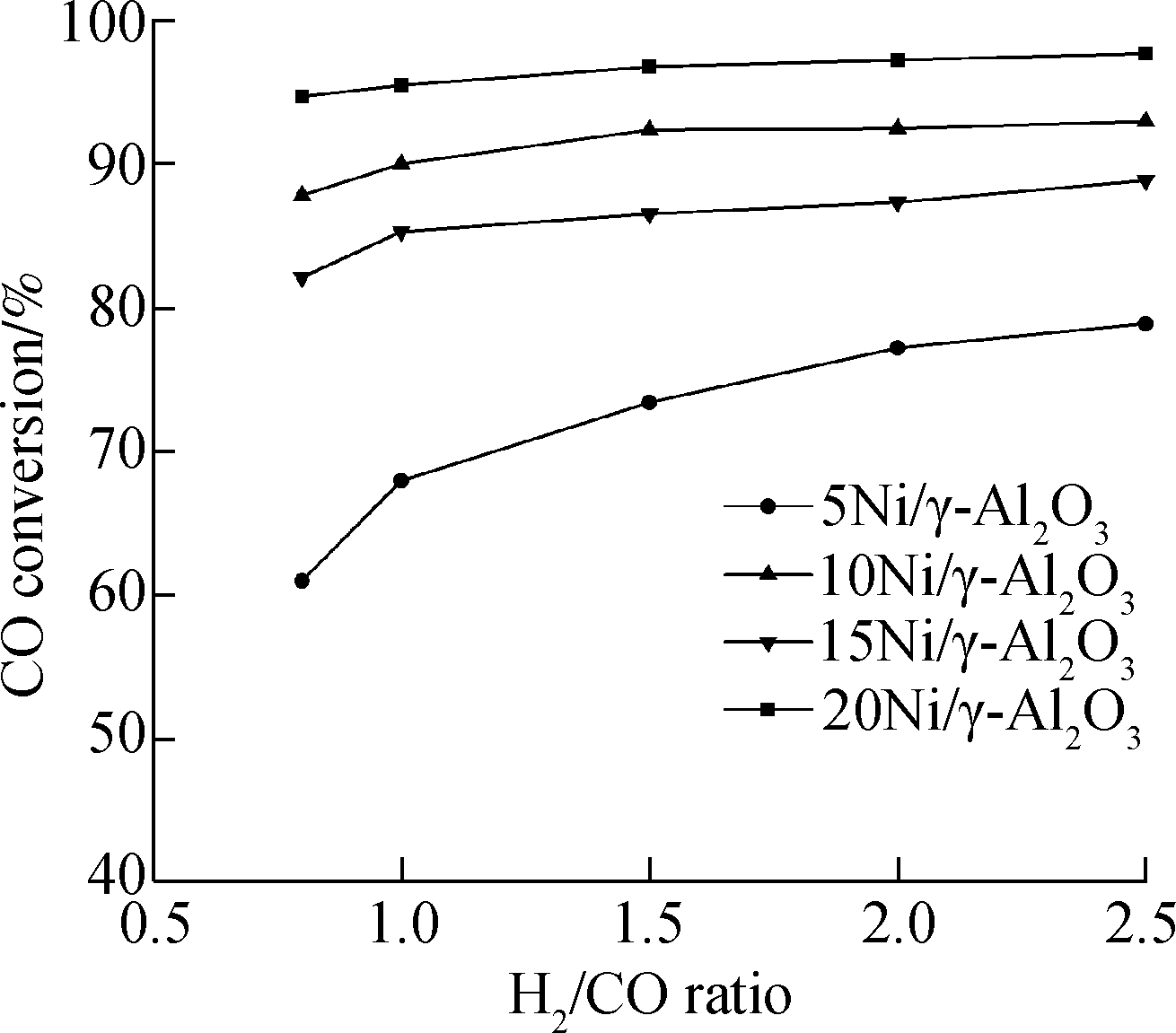
The lines in Fig.8(a) show the varying pattern of CO conversion along with the change of H2/CO ratio. Among them, 20Ni/γ-Al2O3 catalyst has the the highest CO conversion and 5Ni/γ-Al2O3 catalyst has the lowest. Similarly, the CO conversion increases with the increase in the H2/CO ratio for all four catalysts with different Ni loadings. This phenomenon may be due to the fact that a larger H2/CO ratio means more H2 in the reaction system, thus providing more H2 to react with the CO. In terms of CH4selectivity (bar charts in Fig.8(b)), 15Ni/γ-Al2O3 possesses higher CH4selectivity than 10Ni/γ-Al2O3 although the CO conversion of 10Ni/γ-Al2O3 is higher than 15Ni/γ-Al2O3. The combined analysis of CO conversion and CH4selectivity proves that the LHV growth rate using 15Ni/γ-Al2O3 is higher than that using 10Ni/γ-Al2O3 in the H2/CO ratio range of 0.8 to 2.5.
Biomass fuel gas derived during the gasification period generally contains impurities such as tar[27], so washing is commonly employed in order to obtain pure gas product. In this case, dry fuel gas will carry various contents of water according to the washing temperature. In this paper, with the purpose of investigating the effect of water content on the LHV of biomass gasification fuel gas through methanation, several saturation water contents (6.2%, 8.1%, 10.4%, 13.4%, 17.2%) are adopted corresponding to different washing temperatures (45, 50, 55, 60 and 65 ℃). As shown in Fig.9, the LHV growth rate decreases with the water content for both the H2/CO ratio of 2.5 (see Fig.9(a)) and the H2/CO ratio of 1.5 (see Fig.9(b)), which indicates that introducing water into the system plays a negative role in enhancing the LHV of biomass gasification fuel gas.
(a)

(b)
Fig.8 Conversion and selectivity of the catalysts at 350 ℃ with the dry feed gas consisting of 27.0% CO2, 8.0% N2, the H2/CO ratio varying from 0.8 to 2.5, and the liquid water flow rate of 7.63 m/h. (a) CO conversion; (b) CH4 selectivity

(a)

(b)
Fig.9 LHV growth rate of biomass gasification fuel gas after methanation at 300 ℃. (a) The dry feed gas consisting of 46.4% H2, 18.6% CO, 27.0% CO2 and 8.0% N2; (b) The dry feed gas consisting of 39.0% H2, 26.0% CO, 27.0% CO2 and 8.0% N2
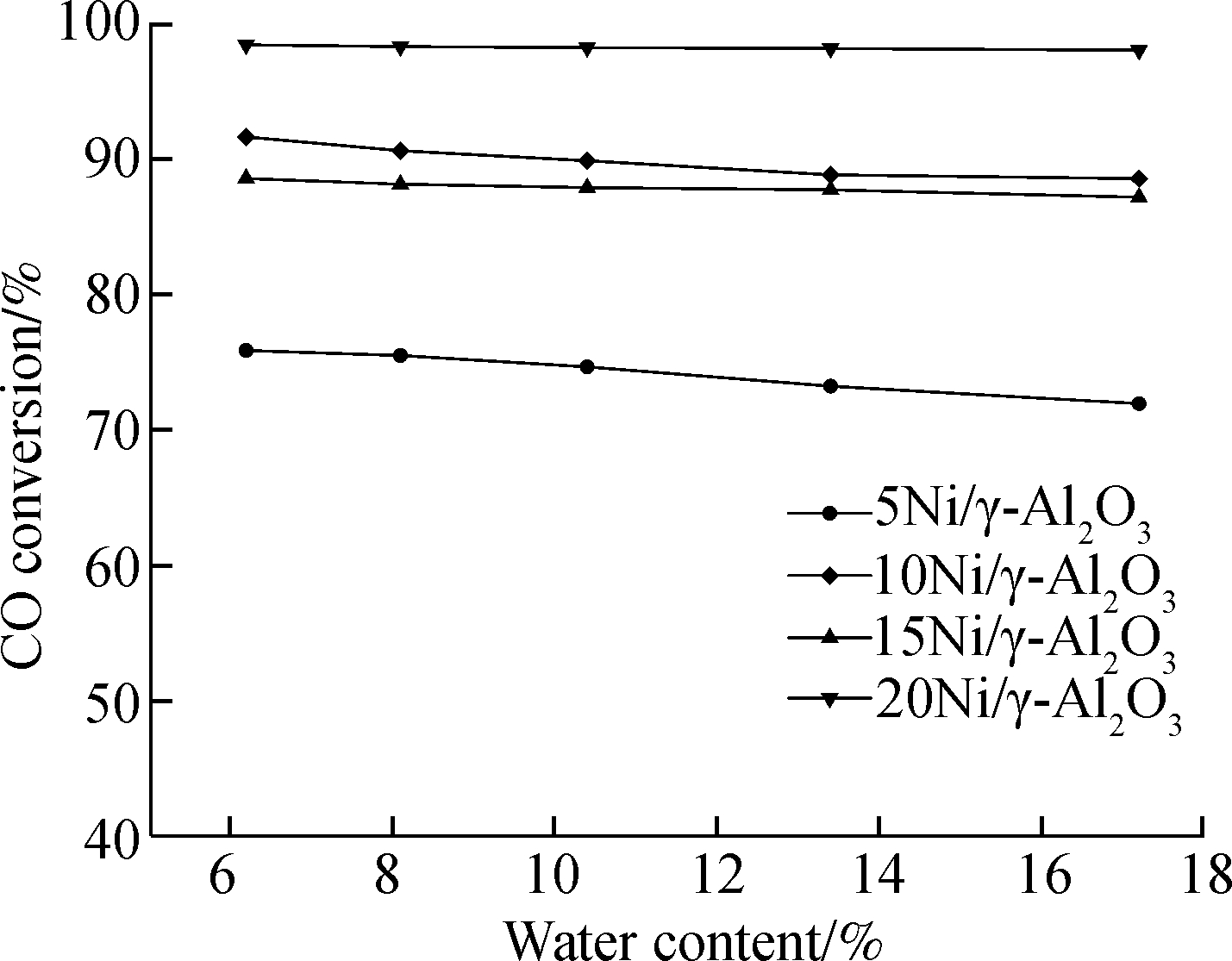
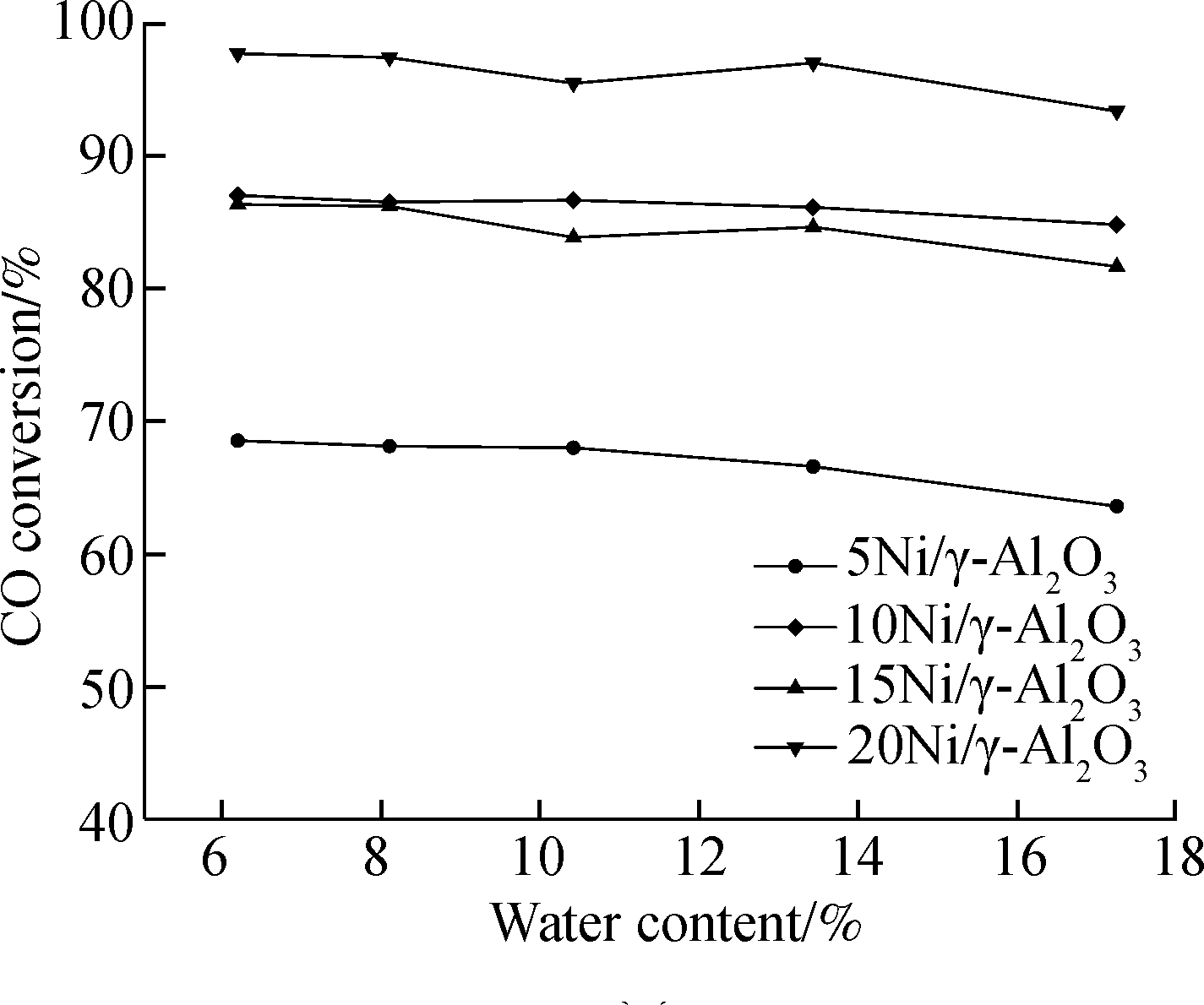
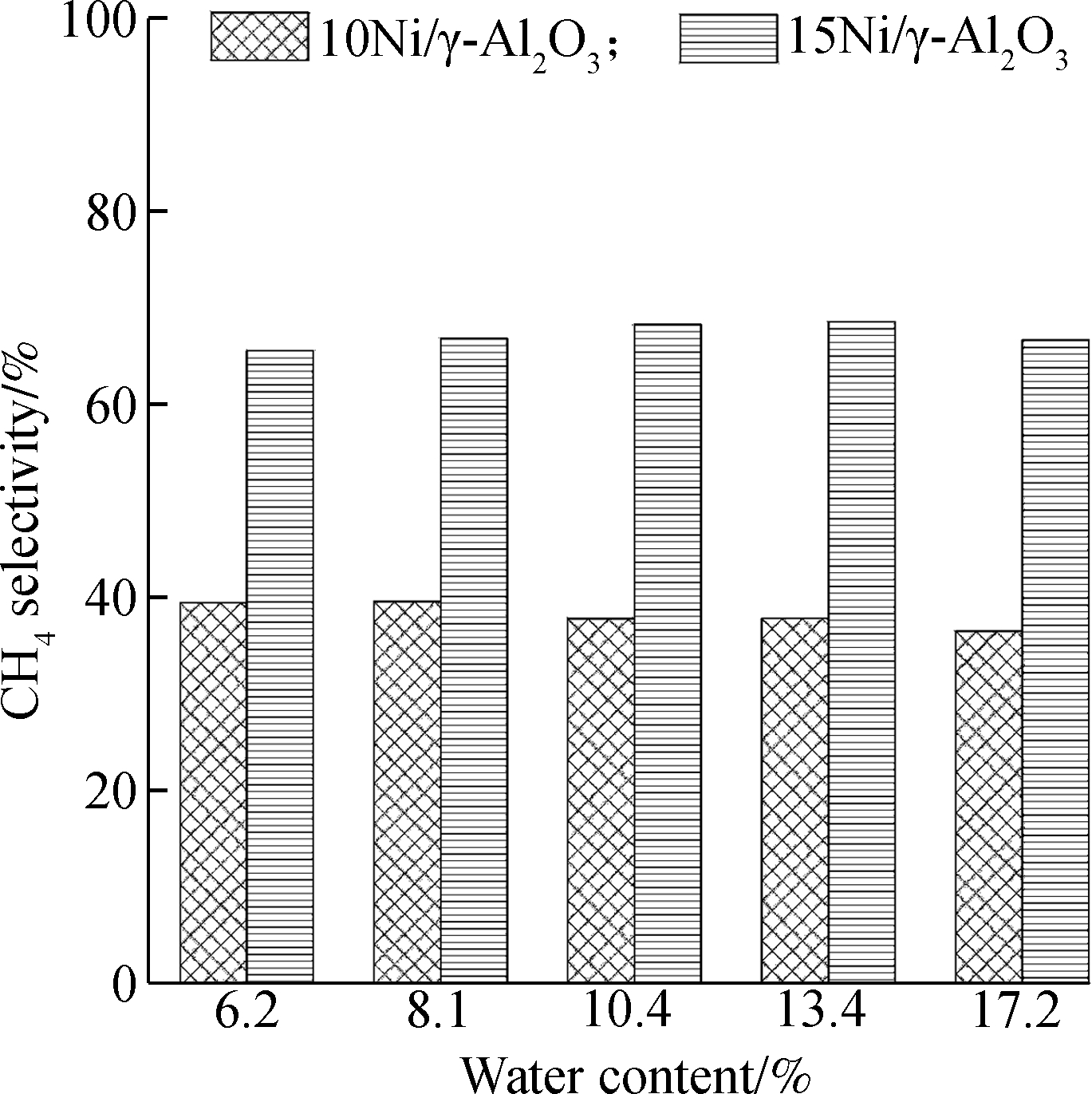
CO conversion is influenced by water content as well. As can be seen in Fig.10(a), CO conversion declines with water content for all four catalysts at the H2/CO ratio of 2.5. This is due to the fact that water is the product of methanation reaction according to Eq.(1) and Eq.(2) and introducing water to the reaction system will increase the partial pressure of water, thus reducing the degree of methanation reaction. As a consequence, CO conversion decreases thereupon. Much attention should be paid on Fig.11(a). For 15Ni/γ-Al2O3 and 20Ni/γ-Al2O3 catalysts, some difference in Fig.10(a) can be seen. The CO conversion increases when the water content increases from 10.4% to 13.4%. This phenomena may be due to water gas shift reaction (WGS) according to Eq.(3). Ni also has a catalytic effect on WGS[28]. When there are abundant Ni active sites for methanation, the extra Ni active sites can be utilized for WGS. This may explain the phenomena mentioned above since there are more Ni active species on the surface of 15Ni/γ-Al2O3 and 20Ni/γ-Al2O3 catalysts than that of 5Ni/γ-Al2O3 and 10Ni/γ-Al2O3 catalysts on the basis of the SEM images in Fig.4. Besides, as mentioned in section 3.3, CH4 selectivity of 15Ni/γ-Al2O3 catalyst is higher than that of 10Ni/γ-Al2O3 catalysts (bar charts shown in Fig.10(b) and Fig.11(b)) though the CO conversion of 15Ni/γ-Al2O3 catalyst is lower than that of 10Ni/γ-Al2O3 catalyst, which leads to the fact that the LHV growth rate using 15Ni/γ-Al2O3 catalyst surpasses the LHV growth rate using 10Ni/γ-Al2O3 catalyst.
(a)

(b)
Fig.10 Conversion and selectivity of the catalysts at 300 ℃ with the dry feed gas consisting of 46.4% H2, 18.6% CO, 27.0% CO2 and 8.0% N2. (a) CO conversion; (b) CH4 selectivity
(a)
(b)
Fig.11 Conversion and selectivity of the catalysts at 300 ℃ with the dry feed gas consisting of 39.0% H2, 26.0% CO, 27.0% CO2 and 8.0% N2. (a) CO conversion; (b) CH4 selectivity
1) The LHV of biomass gasification fuel gas can be heightened to various degrees with the catalysis of Ni/γ-Al2O3 under distinct operation conditions. According to the performance tests, 15Ni/γ-Al2O3 and 20Ni/γ-Al2O3 are highly efficient in terms of improving the LHV of biomass gasification fuel gas due to their remarkable catalytic effect.
2) When investigating the single influential factor, 15Ni/γ-Al2O3 and 20Ni/γ-Al2O3 catalysts function desirably at 350 ℃ due to their satisfactory CO conversion and CH4 selectivity. Also, a higher H2/CO ratio and lower water content are favorable for elevating the LHV of biomass gasification fuel gas.
3) From the results of the experiments, improving the LHV of biomass gasification fuel gas with a low H2/CO ratio (<1.0) merely through methanation is difficult to achieve. Adding a process of water gas shift reaction before methanation or employing catalysts with both functions may address such a problem.
References
[1]Hosseini S E, Wahid M A. Development of biogas combustion in combined heat and power generation [J]. Renewable and Sustainable Energy Reviews, 2014, 40: 868-875. DOI:10.1016/j.rser.2014.07.204.
[2]Pérez N P, Machin E B, Pedroso D T, et al. Biomass gasification for combined heat and power generation in the Cuban context: Energetic and economic analysis [J]. Applied Thermal Engineering, 2015, 90: 1-12. DOI:10.1016/j.rser.2014.07.204.
[3]Biagini E, Barontini F, Tognotti L. Development of a bi-equilibrium model for biomass gasification in a downdraft bed reactor [J]. Bioresource Technology, 2016, 201: 156-165. DOI:10.1016/j.biortech.2015.11.057.
[4]Berrueco C, Recari J, Abelló S, et al. Experimental investigation of solid recovered fuel (SRF) gasification: Effect of temperature and equivalence ratio on process performance and release of minor contaminants [J]. Energy & Fuels, 2015, 29(11): 7419-7427. DOI:10.1021/acs.energyfuels.5b02032.
[5]Kwapinska M, Xue G, Horvat A, et al. Fluidized bed gasification of torrefied and raw grassy biomass (miscanthus×gigantenus). The effect of operating conditions on process performance [J]. Energy & Fuels, 2015, 29(11): 7290-7300. DOI:10.1021/acs.energyfuels.5b02032.
[6]Biagini E, Barontini F, Tognotti L. Gasification of agricultural residues in a demonstrative plant: Vine pruning and rice husks [J]. Bioresource Technology, 2015, 194: 36-42. DOI:10.1016/j.biortech.2015.07.016.
[7]Barisano D, Canneto G, Nanna F, et al. Steam/oxygen biomass gasification at pilot scale in an internally circulating bubbling fluidized bed reactor [J]. Fuel Processing Technology, 2016, 141: 74-81. DOI:10.1016/j.fuproc.2015.06.008.
[8]Balu E, Lee U, Chung J N. High temperature steam gasification of woody biomass—A combined experimental and mathematical modeling approach [J]. International Journal of Hydrogen Energy, 2015, 40(41): 14104-14115. DOI:10.1016/j.ijhydene.2015.08.085.
[9]Yu H, Li Z, Yang X, et al. Experimental research on oxygen-enriched gasification of straw in an entrained-flow gasifier [J]. Journal of Renewable and Sustainable Energy, 2013, 5(5): 053127. DOI:10.1063/1.4822260.
[10]Wang Z, He T, Qin J, et al. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier [J]. Fuel, 2015, 150: 386-393. DOI:10.1016/j.fuel.2015.02.056.
[11]Kopyscinski J, Schildhauer T J, Biollaz S M A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009 [J]. Fuel, 2010, 89(8): 1763-1783. DOI:10.1016/j.fuel.2010.01.027.
[12]Rönsch S, Schneider J, Matthischke S, et al. Review on methanation—From fundamentals to current projects [J]. Fuel, 2016, 166: 276-296. DOI:10.1016/j.fuel.2015.10.111.
[13]Kim W, Koo K Y, Lee H J, et al. Highly dispersed nickel catalyst promoted by precious metals for CO selective methanation [J]. International Journal of Hydrogen Energy, 2015, 40(32): 10033-10040. DOI:10.1016/j.ijhydene.2015.06.033.
[14]Tada S, Kikuchi R, Wada K, et al. Long-term durability of Ni/TiO2 and Ru-Ni/TiO2 catalysts for selective CO methanation [J]. Journal of Power Sources, 2014, 264: 59-66. DOI:10.1016/j.ijhydene.2015.06.033.
[15]Ma S, Tan Y, Han Y. Water-gas shift coupling with methanation over MOx modified nanorod-NiO/γ-Al2O3 catalysts [J]. Journal of Industrial and Engineering Chemistry, 2011, 17(4): 723-726. DOI:10.1016/j.jiec.2011.05.014.
[16]Kiendl I, Klemm M, Clemens A, et al. Dilute gas methanation of synthesis gas from biomass gasification [J]. Fuel, 2014, 123: 211-217. DOI:10.1016/j.fuel.2014.01.036.
[17]Gao J J, Liu Q, Gu F N, et al. Recent advances in methanation catalysts for the production of synthetic natural gas [J]. RSC Advances, 2015, 5(29): 22759-22776. DOI:10.1039/c4ra16114a.
[18]Zhou G, Wu T, Zhang H, et al. Carbon dioxide methanation on ordered mesoporous Co/KIT-6 catalyst [J]. Chemical Engineering Communications, 2014, 201(2): 233-240. DOI:10.1080/00986445.2013.766881.
[19]Abdel-Mageed A M, Eckle S, Behm R J. High selectivity of supported Ru catalysts in the selective CO methanation-water makes the difference [J]. Journal of the American Chemical Society, 2015, 137(27): 8672-8675. DOI:10.1021/jacs.5b03689.
[20]Escobar M, Gracia F, Karelovic A, et al. Kinetic and in situ FTIR study of CO methanation on a Rh/Al2O3 catalyst [J]. Catalysis Science & Technology, 2015, 5(9): 4532-4541. DOI:10.1039/c5cy00676g.
[21]Lu X, Gu F, Liu Q, et al. VOx promoted Ni catalysts supported on the modified bentonite for CO and CO2 methanation [J]. Fuel Processing Technology, 2015, 135: 34-46. DOI:10.1016/j.fuproc.2014.10.009.
[22]Liu Y, Zhu L, Wang X, et al. Catalytic methanation of syngas over Ni-based catalysts with different supports [J]. Chinese Journal of Chemical Engineering, 2017, 25(5): 602-608. DOI:10.1016/j.cjche.2016.10.019.
[23]Ding M, Tu J, Wang T, et al. Bio-syngas methanation towards synthetic natural gas (SNG) over highly active Al2O3-CeO2 supported Ni catalyst [J]. Fuel Processing Technology, 2015, 134: 480-486. DOI:10.1016/j.fuproc.2015.03.006.
[24]Meshkani F, Rezaei M. Mesoporous Ba-promoted chromium free Fe2O3-Al2O3-NiO catalyst with low methanation activity for high temperature water gas shift reaction [J]. Catalysis Communications, 2015, 58: 26-29. DOI:10.1016/j.catcom.2014.08.028.
[25]Mei Z, Li Y, Fan M, et al. The effects of bimetallic Co-Ru nanoparticles on Co/RuO2/Al2O3 catalysts for the water gas shift and methanation [J]. International Journal of Hydrogen Energy, 2014, 39(27): 14808-14816. DOI:10.1016/j.ijhydene.2014.07.072.
[26]Bai X, Wang S, Sun T, et al. The sintering of Ni/Al2O3 methanation catalyst for substitute natural gas production [J]. Reaction Kinetics, Mechanisms and Catalysis, 2014, 112(2): 437-451. DOI:10.1007/s11144-014-0700-8.
[27]Chen G Y, Liu C, Ma W C, et al. Catalytic cracking of tar from biomass gasification over a HZSM-5-supported Ni-MgO catalyst [J]. Energy & Fuels, 2015, 29(12): 7969-7974. DOI:10.1021/acs.energyfuels.5b00830.
[28]Arbel ez O, Reina T R, Ivanova S, et al. Mono and bimetallic Cu-Ni structured catalysts for the water gas shift reaction [J]. Applied Catalysis A: General, 2015, 497: 1-9. DOI:10.1016/j.apcata.2015.02.041.
ez O, Reina T R, Ivanova S, et al. Mono and bimetallic Cu-Ni structured catalysts for the water gas shift reaction [J]. Applied Catalysis A: General, 2015, 497: 1-9. DOI:10.1016/j.apcata.2015.02.041.
摘要:制备了负载于γ-Al2O3载体上的镍基甲烷化催化剂用于提高生物质气化燃气的低位热值.在性能测试之前,采用氮气等温吸附/脱附、XRD和SEM等方法对催化剂样品进行表征.一系列催化性能测试表明,在提高生物质燃气低位热值方面,负载量为15 %和20 %的镍基催化剂较负载量为5 %和10 %的镍基催化剂具有更优越的催化甲烷化性能.此外,反应温度、H2/CO比和含水量等可控影响因素在生物质燃气甲烷化过程中影响很大.15Ni/γ-Al2O3和20Ni/γ-Al2O3催化剂在350 ℃时具有更高的CO转化率和CH4选择性并且在此温度下生物质燃气的低位热值增长率可达34.3 %.综合考虑CO转化率和CH4选择性,由于较高的H2/CO比和较低的含水量有利于提高甲烷化的反应程度,因此,增大H2/CO比和降低含水量有利于提高生物质燃气的低位热值.
关键词:镍基催化剂;甲烷化;生物质燃气;低位热值
中图分类号:O643.3
DOI:10.3969/j.issn.1003-7985.2017.04.010
Received 2017-05-19,
Revised 2017-09-04.
Biographies:Dong Xinxin (1991—), male, graduate; Jin Baosheng (corresponding author), male, professor, bsjin@seu.edu.cn.
Foundation item:The International S&T Cooperation Program of China (No.2014DFE70150).
Citation:Dong Xinxin, Jin Baosheng, Wang Yanyan, et al. Experiments on Ni/γ-Al2O3catalyst for improving lower heating value of biomass gasification fuel gas via methanation[J].Journal of Southeast University (English Edition),2017,33(4):448-456.
DOI:10.3969/j.issn.1003-7985.2017.04.010.