Cell is the basic unit of structure and function of organisms, and plays an important role in metabolism, signal transduction and other life activities[1]. It is well-known that cellular activities such as cell proliferation, gene expression, and enzyme reaction can be reflected in cellular temperature changes[2-4]. Researchers have made numerous attempts in the exploration of intracellular temperature[5-6]. However, given that the scale of temperature is relatively small, it is difficult to measure the single cellular temperature accurately. With the development of nanotechnology, the process of studying real-time intracellular temperature measurement at the micro/nano scale has been accelerated and many effective approaches have emerged[7]. Luminescence-based thermometry has emerged as one of the principal means of measuring intracellular temperature[8]. For example, Gota et al.[9]developed a highly hydrophilic fluorescent nanogel thermometer to detect COS7 cells’ temperature. Okabe et al.[10] demonstrated the first intracellular temperature mapping based on a  uorescent polymeric thermometer and
uorescent polymeric thermometer and  uorescence lifetime imaging microscopy. Suzuki et al.[11] used a similar dual-channel approach with two glass micropipettes filled with the thermosensitive fluorescent dye Europium (Ⅲ) thenoyltrifluoroacetonate trihydrate to detect the change in cell temperature by controlling the enzymatic activity of Ca2+-ATPases in the endoplasmic reticulum in a single Hela cell. Although fluorescence-based methods have good spatial resolution, the potential toxicity of materials to cells and low measurement accuracy remains elusive.
uorescence lifetime imaging microscopy. Suzuki et al.[11] used a similar dual-channel approach with two glass micropipettes filled with the thermosensitive fluorescent dye Europium (Ⅲ) thenoyltrifluoroacetonate trihydrate to detect the change in cell temperature by controlling the enzymatic activity of Ca2+-ATPases in the endoplasmic reticulum in a single Hela cell. Although fluorescence-based methods have good spatial resolution, the potential toxicity of materials to cells and low measurement accuracy remains elusive.
To date, thermocouple has been widely used in temperature measurement because of its superior characteristics in stability, accuracy and practicality.Based on our previous works, micro-nano thermocouple had the advantage of high precision and quick time response to measure temperature[12-15].Thermocouple (TC) is composed of tungsten (W), polyurethane (PU) and platinum (Pt)[12]. The functional part of the TC is a W-Pt junction based on the Seebeck effect. According to the Seebeck effect, when the ends of two different metals are joined together to form a loop and the temperature of the two contact points differs, a current and a corresponding electromotive force will appear in the loop. During temperature measurement, one contact point of the thermocouple needs to be placed at known and constant temperature, and the temperature of the other contact point can be obtained by calculating the temperature difference of the two contact points. The temperature obtained by a single TC cannot eliminate the interference caused by environmental factors, and the extended circuit of the metal junction also introduces errors[13]. To address the above problems, we developed a dual-thermocouple difference thermometry method. This method improved the connection mode of thermocouple and removed the setting of reference temperature. The relative temperature difference between the cells and the culture medium was detected by the difference method, which reflected changes in cell temperatures that were not disturbed by the environment.
Cell behaviour greatly varies in metabolic activities to meet nutrition and energy requirements, while the difference in metabolic activities may lead to a difference in cell temperature[16-17]. Cancer cells usually have more pronounced temperature characterization than normal cells due to their increased metabolic activity[18-19]. For a more practical use of the dual-thermocouple difference method, we chose glioma cells (U251) for the temperature measurement and observed changes in temperature. Two TCs were placed in the cell and its culture medium, respectively, to detect the changes in cell temperature. The signal generated on the TC was displayed on the computer through amplification, filtering and other processing. In the detection of small temperature fluctuations, the results obtained by the dual thermocouple difference method are more convincing. Therefore, the dual thermocouple difference method is potentially a highly powerful technique for the real-time measurement of intracellular temperature changes. It provides a new dimension to researching cancer cells by monitoring heat production. More comprehensive thermal information in cell physiology obtained may help us understand the mechanism of cellular thermogenesis[20].
In this work, we developed a dual-thermocouple difference method to measure intracellular temperature. The interference of the reference point was eliminated by the difference measurement of two TCs. The human glioma cells (U251) were seeded in a 35 mm culture dish, and then the changes of cell temperature were recorded. It suggested that our method was able to detect tiny cellular temperature changes. Compared with single thermocouple temperature measurement, a dual-thermocouple difference method reduced the error sources and improved the anti-interference ability. It will drive the development of cell temperature measurement at the single-cell level.
1 Experimental Method
We designed the dual-thermocouple difference method and tested its feasibility, seeded human glioma cells (U251) in a 35 mm culture dish, and evaluated the temperature response of the cell. We also tried to measure intracellular temperature using a conventional single thermocouple. The advantages of the dual thermocouple difference method were proved by comparing the results of temperature measurement. The schematic diagram of the experiment is shown in Fig.1(a).
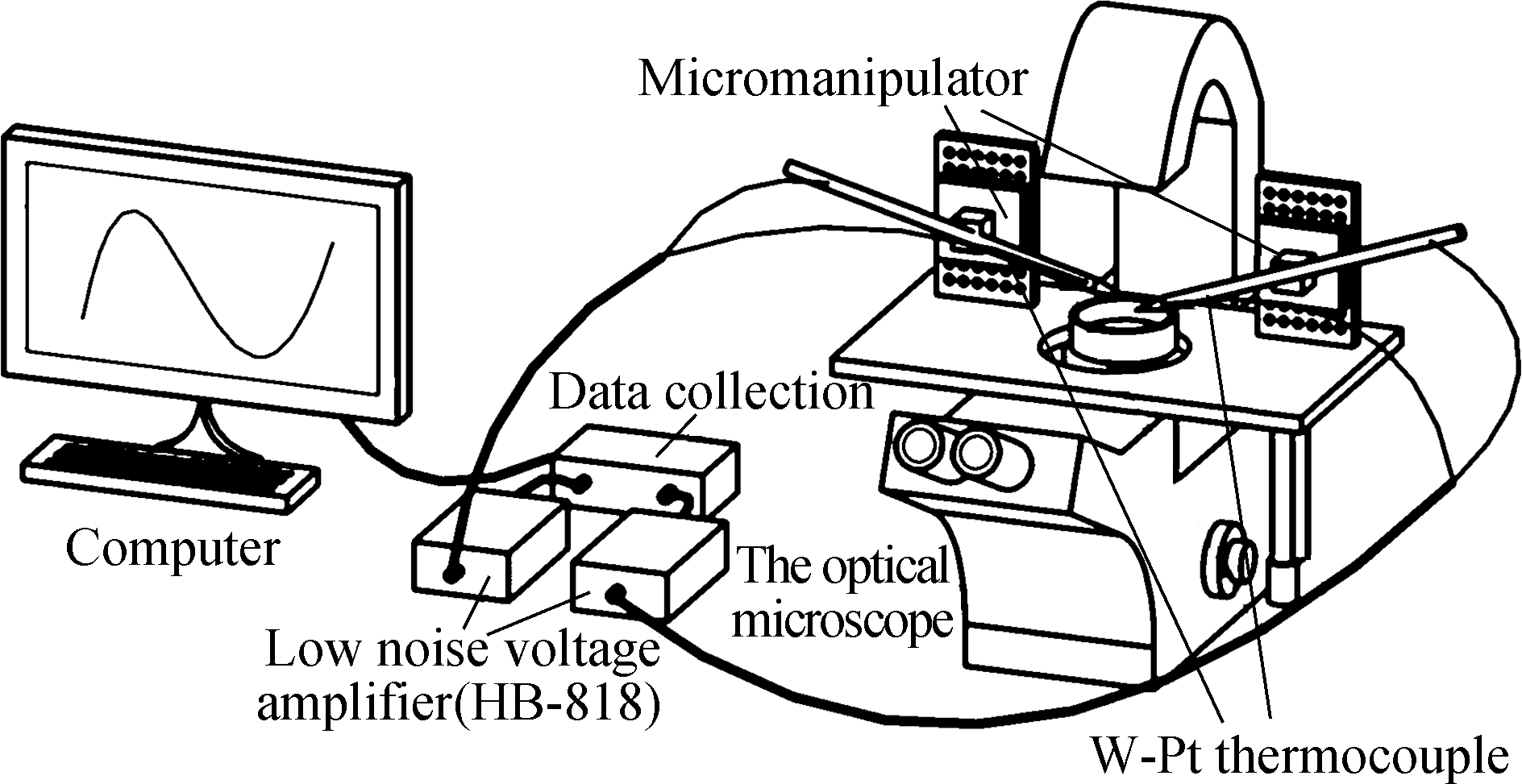
(a)
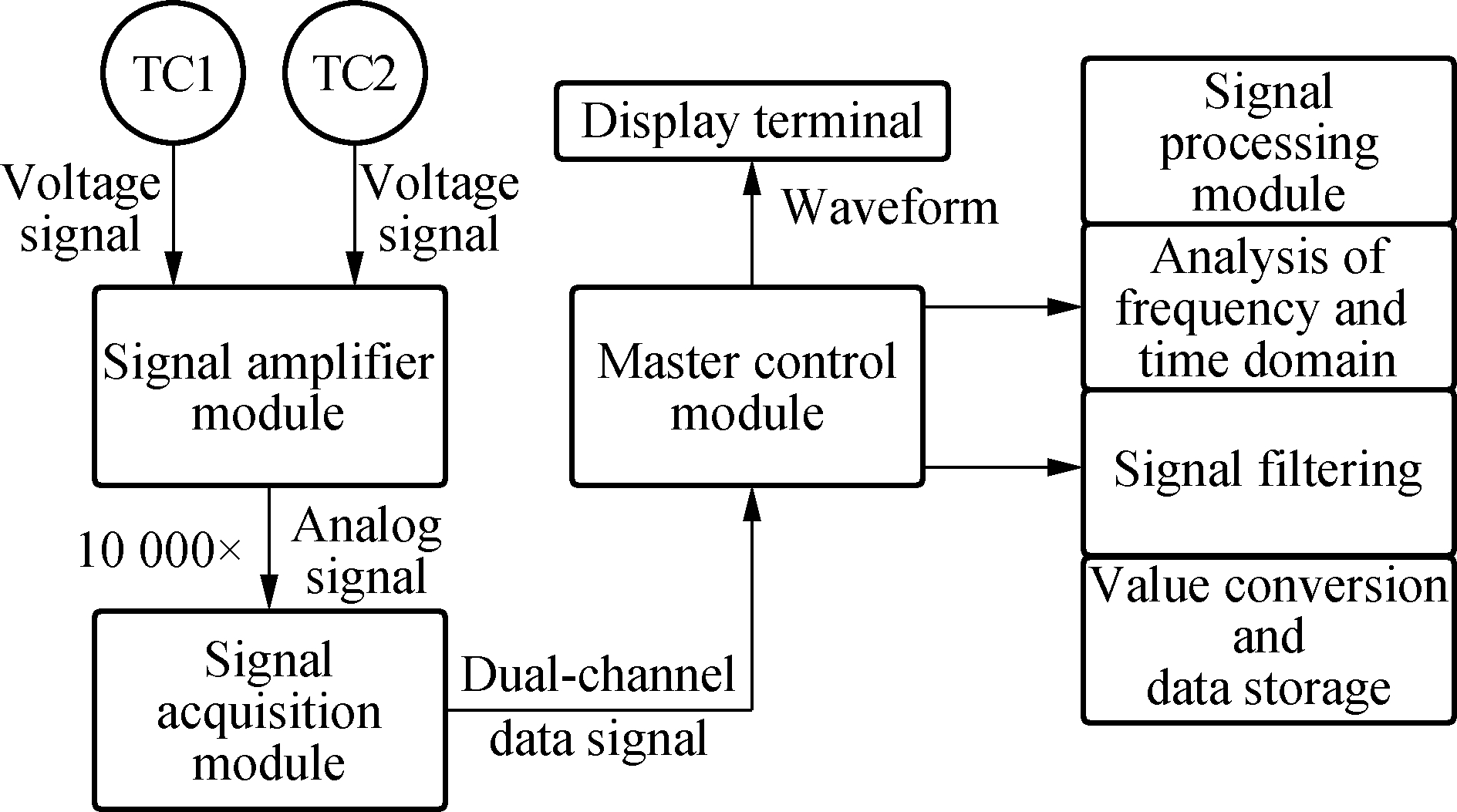
(b)Fig.1 Schematic diagram of experimental setup and process design. (a) Intracellular temperature measuring device based on the dual-thermocouple difference method; (b) System flowchart
1.1 Device building
The device consisted of the operating platform and the signal acquisition module. The operating platform for cellular experiments consisted of two TCs, an inverted microscope (Axiovert 200, ZEISS) and a micromanipulator (PCS-5000, Burleigh). The signal acquisition module was composed of two-low noise, low-drift, and broadband voltage amplifiers (HB-818, Hongbin), a multi-channel digital acquisition card (MyDAQ NI) and a computer to process data. Two TCs were fixed to the micromanipulator by a self-made fixture, and the W-Pt junctions were connected to the voltage amplifier. With the help of the microscope and the micromanipulator, TCs can be moved to any single cell in the field of view. The schematic diagram of the device is shown in Fig.1(a). Since the scale of temperature inside cells was very small, the signals on the TCs were collected after being amplified 10 000 times by an amplifier and converted into the cell temperature by signal processing. Fig.1(b) presents the system structure chart. This device had some important functions such as multichannel continuous data acquisition, parameter settings, and a waveform display, which met the testing requirements.
1.2 Fabrication of the TCs
Tungsten was a suitable material used as the base of various probes. Platinum acted as the outer pole due to its good biocompatibility, and polyurethane was used as an insulation between W and Pt. The tungsten rod (length: 70 mm; diameter: 0.3 mm) was completely immersed into anhydrous ethanol to the remove industrial oils that adhered to the surface before electrochemical etching. The depth of the tungsten rod in the etching solution was adjusted to ensure that the tip of the tungsten rod had a curvature radius of less than 200 nm and a cone angle of between 10° and 20°. The tungsten rod with a standard shape was selected using an inverted microscope (Axiovert 200, ZEISS), and then wrapped in 60 g/L PU/THF dissolvent solution. The tungsten’s tip was exposed due to the liquid level tension. The W-treated rod surface was splattered with platinum by using a vacuum splatter apparatus (Q150TS, Quorum) to make TCs.
1.3 The principle of the difference thermometry
The key to measuring temperature with TCs was to determine the relationship between temperature and voltage. When Seebeck values for metals were constant with a small temperature range (20-40 ℃), the thermoelectric potential can be obtained based on the following formula:
V=![]() (SA(T)-SB(T))dT=S(Ttest-Tref)
(SA(T)-SB(T))dT=S(Ttest-Tref)
(1)
where V is the voltage; S=SA(T)-SB(T) is defined as the thermoelectric power; Ttest is the temperature of the W-Pt junction; and Tref is the temperature of the cold junction. When the cold junctions of two TCs were placed together, the difference in temperature (ΔT) between the two W-Pt junctions can be calculated with
Vtip1=Stip1(Ttest1-Tref1)
(2)
Vtip2=Stip2(Ttest2-Tref2)
(3)
When Tref1=Tref2,
(4)
where Vtip1, Vtip2 are the voltages of the two TCs; Stip1, Stip2 are their thermoelectric power; ΔT is the relative temperature change of a single cell in its culture medium.
Therefore, ΔT between the two TCs can be obtained through their voltage values and their Seebeck coefficients.
1.4 Device calibration
In the signal processing module, the effective signal frequency band was screened by the frequency domain analysis. The butterworth filter from the LabVIEW program was used to eliminate the noise interference. In order to determine the device accuracy, the voltage value from 5 μV to 30 μV which was generated by a micro-signal generator, was measured by the device and the high precision digital multimeter (precision: 0.3 μV) at the same time. The voltage deviations were obtained by comparing the test results of our device and the high precision digital multimeter. Then, the voltage deviations of the device were corrected by software compensation.
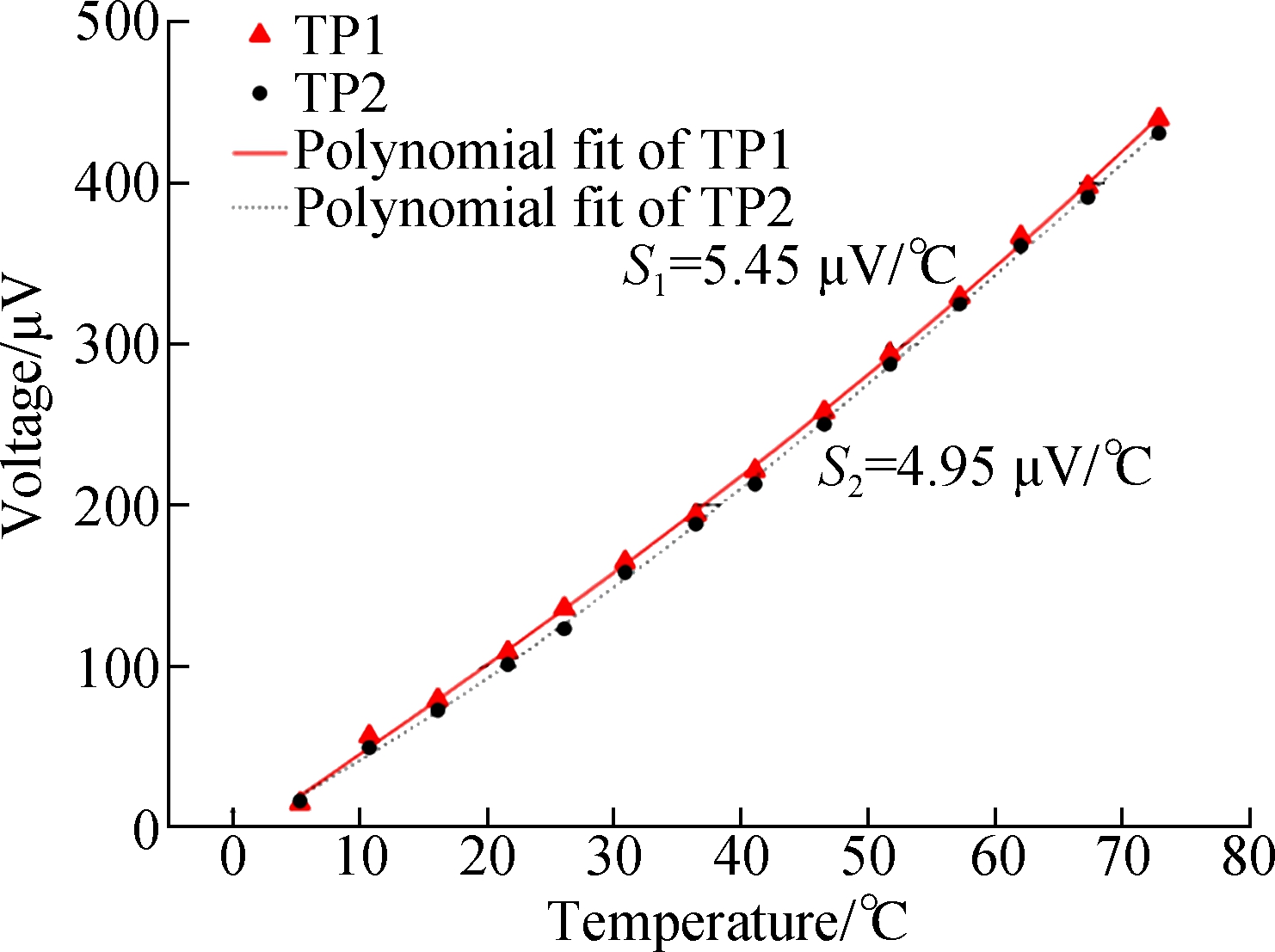
The property of each thermocouple was not consistent due to manufacturing process deviations. Thus, a calibration for each thermocouple to be used is necessary. The calibration to TC was conducted in a standard thermally gradient distilled-water bath. The water was heated from 5 ℃ to 70 ℃ and the W-Pt junctions of the TCs were submerged in it. The voltage of the TC varied with the temperature and was recorded every 5 ℃ using a high precision digital multimeter (Agilent 34410A). The formula of voltage signals versus temperatures can be obtained through polynomial fitting.
To simulate the cell culture environment, 2 mL of medium was added into a 35 mm dish while two TCs were simultaneously transferred into the medium. The tips of TCs were kept as close as possible without damaging it, and the temperature at the same point was measured.
1.5 Cell culture
The human glioma cell line (U251) was obtained from KeyGENBioTECH (Nanjing, China). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS,Cellsera) and maintained at 37 ℃ in 5% CO2.
1.6 Cell temperature measurement
Cells grown in 35 mm dishes were placed on the objective platform of the microscope and the two TCs were controlled by the micromanipulator to measure the temperature. The two TCs were moved to the tested cell and remained in the current state until the reading was stable. One was gently touched with the cell as a test thermometer, and the other was placed near the cell as a reference thermometer.
2 Results and Discussion
2.1 Device realization
We built a dual-thermocouple difference thermometry device and the connection mode of TCs was simplified. During the use of a single TC, multiple junctions (J1, J2, J3, and J4) were generated due to the connection of the thermocouple and the metal conductor. In order to capture the cellular temperature, the cold junction (J2) should be placed in a mixture of ice and water at a constant temperature. The contact point between the thermocouple and the conductor (J3 and J4) should be maintained at the same temperature (see Fig.2(a)). The wire connection needs to be in an isothermal state during the course of use. The experimental conditions were difficult to realize, and incorrect operation could produce unwanted errors. In the dual-thermocouple mode (see Fig.2(b)), the two cold junctions are connected at the same temperature, so ΔT between the two TCs can be obtained through Eq.(1). There was no need to set the condition of constant temperature, and the measurement results were completely comprised of the temperature difference between the cell and its culture medium.
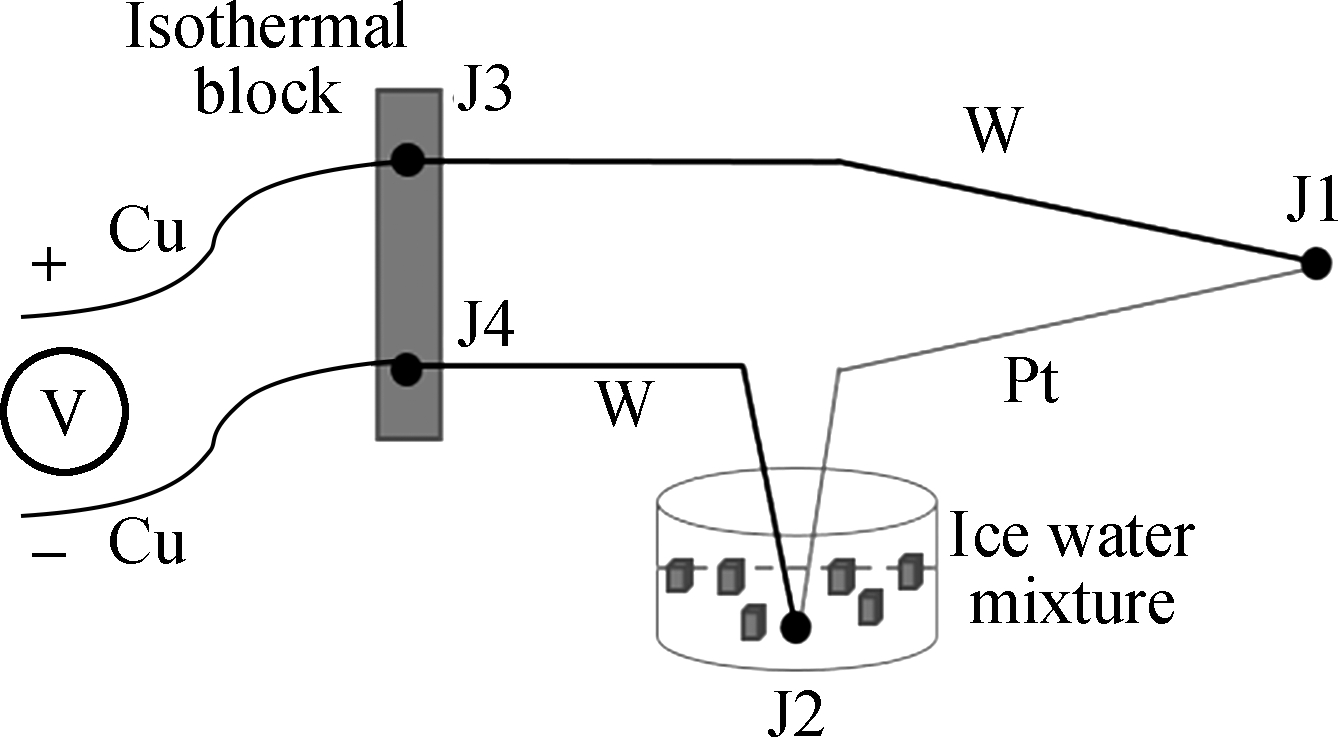
(a)
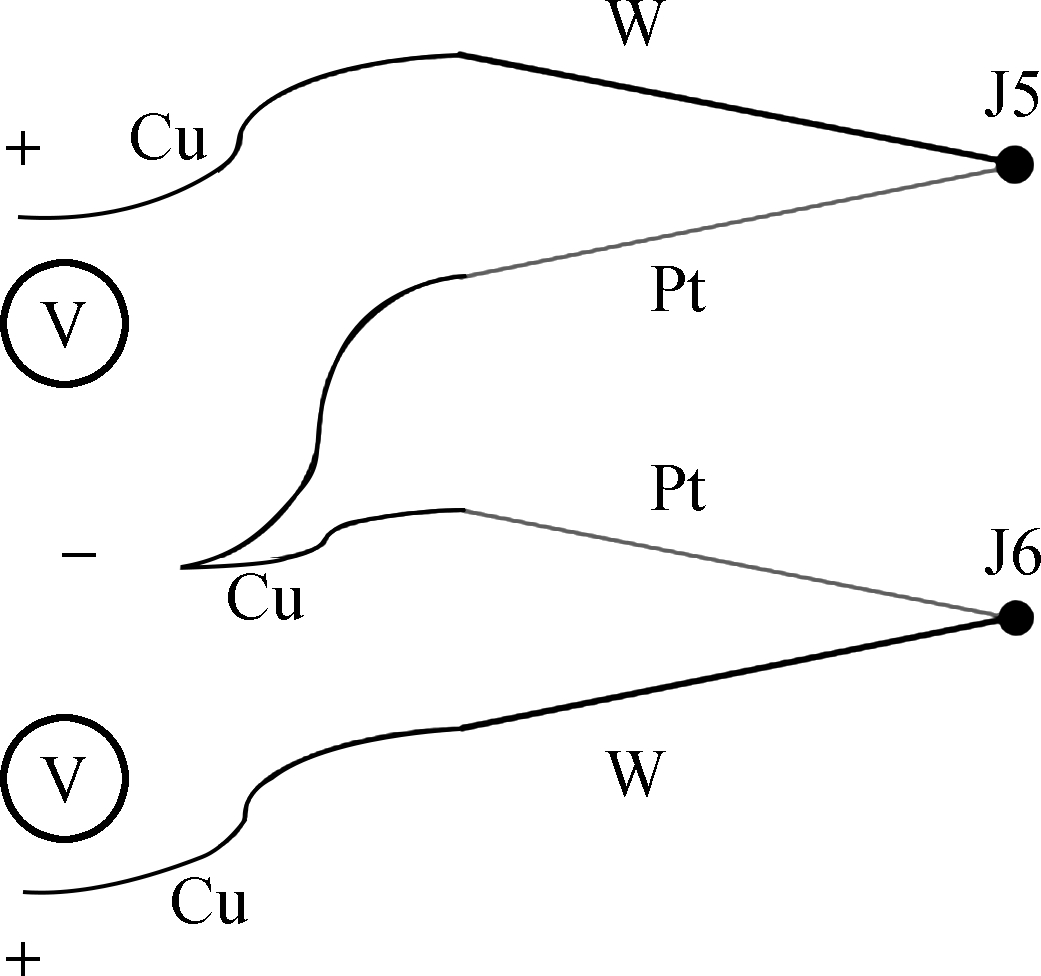
(b)Fig.2 Comparison of single-thermocouple connection mode and dual-thermocouple connection mode. (a) Single-thermocouple mode; (b) Dual-thermocouple mode
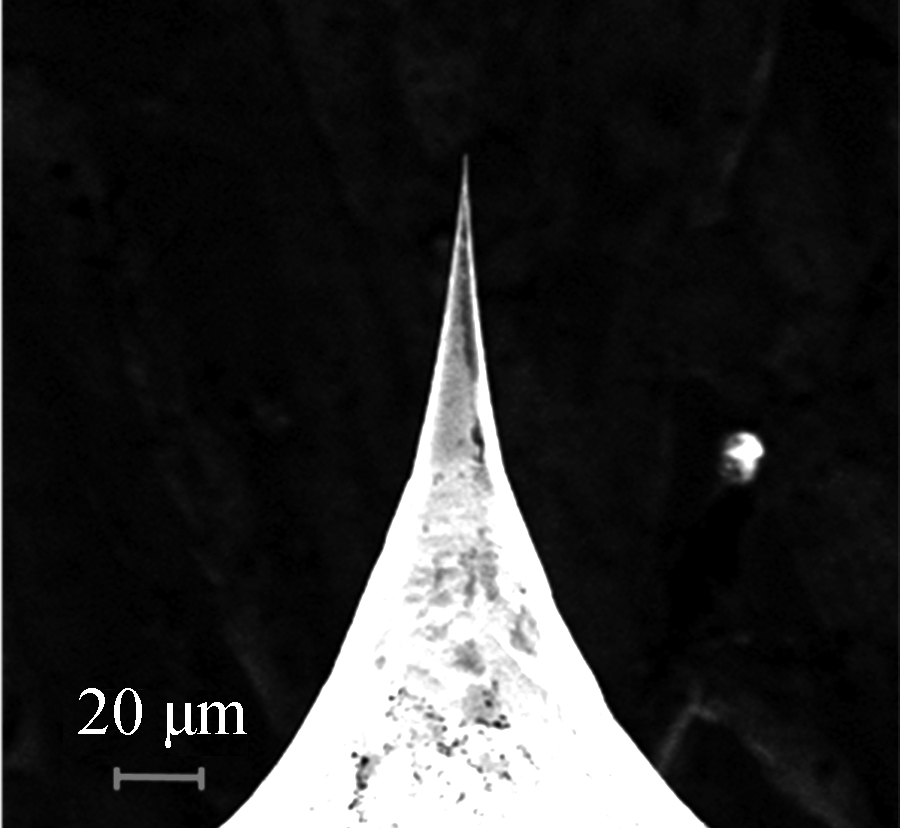
The structure of the TC is shown in Fig.3(a). Needle-like morphology made the W-Pt junction smaller and more suitable for single-cell analysis. In the magnified image, the tip of the thermocouple was less than 1 μm which indicated that the TC could meet the requirements of single cell temperature measurements (see Fig.3(b)).

(a)
(b)
Fig.3 Schematic diagram of the TC. (a) Schematic diagram of the TC structure; (b) SEM images of the tungsten rod after electrochemical etching (800×)
2.2 Signal processing
The purpose of the frequency domain analysis was to make the signal as smooth as possible while retaining the characteristics of the signal. When the low frequency signal was submerged in the high frequency noise, as long as the signal and noise were in different frequency bands, a suitable frequency selective filter could be designed to filter the high frequency noise. Fig.4(a) shows the fluctuation of the signal in different frequency bands. It was found that the signal was stable and accurate in the low frequency range (0-50 Hz). The original signal showed good stability after being filtered. Fig.4(b) shows the result of signal filtering that the signal fluctuation range was less than 0.5 μV (the red line). One of the advantages of the virtual instrument based on LabVIEW was that the filter parameters can be adjusted according to the actual situation to ensure the optimum performance of the device.
The device was calibrated with the data collected by the high precision digital multimeter as a reference, and the device accuracy was 0.5 μV in a low voltage range (0-15 μV) (see Fig.4(c)). The voltage in this range was converted to temperature, far exceeding the heat produced by a single cell, which met the requirements of cellular temperature measurement.

(a)

(b)
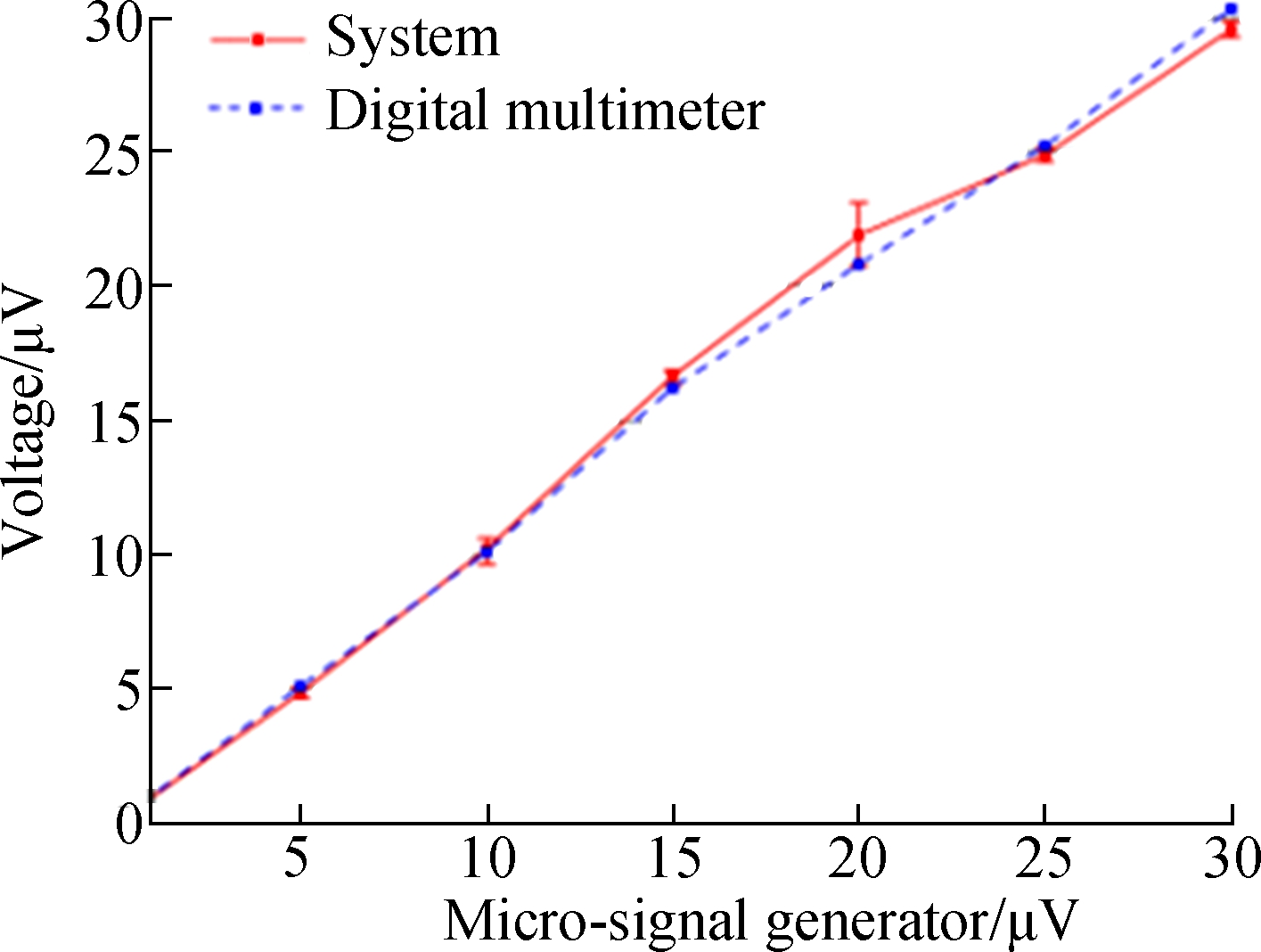
(c)Fig.4 Signal processing results. (a) Results of frequency domain analysis; (b) The curves of the voltage signal before and after filtering; (c) The test curves of the voltages provided by the system and the high precision digital multimeter
2.3 Device test
As shown in Fig.5(a), the relationship between voltage and temperature obtained by the trinomial fitting in the calibration process of TC was clear. S was the Seebeck coefficient of the TC. According to the Seebeck coefficient of 5 μV/℃, the temperature precision was about 0.1 ℃. The two TCs with known Seebeck coefficients were combined with the signal acquisition module in accordance with Fig.1(a). The temperature of the same point was measured by two TCs. Fig.5(b) shows the voltage value of each thermocouple and the temperature value calculated by Eq.(4). Although the voltage values from each TC showed an obvious fluctuation, the fluctuation trend was parallel, and the relative temperature obtained through the device was stabilized and close to zero. Compared with single thermocouple, the dual-thermocouple difference method showed better accuracy and anti-interference ability.
(a)
(b)Fig.5 TCs calibration results and device test results. (a) Voltage versus the temperature of the TCs; (b) The test curve of the dual-thermocouple difference method
2.4 Comparison of single-thermocouple mode and dual-thermocouple mode
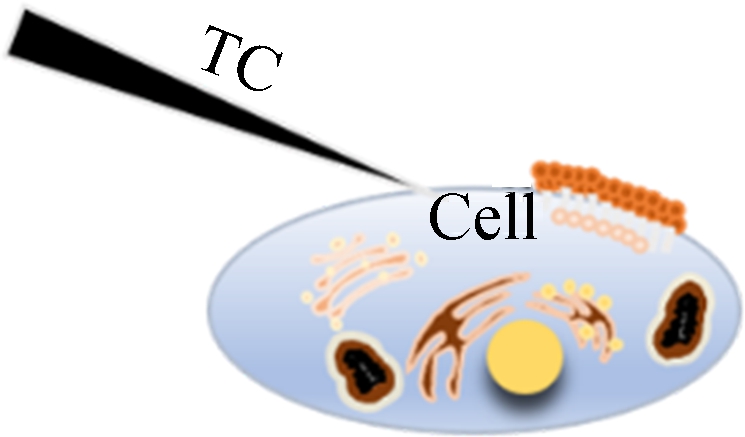
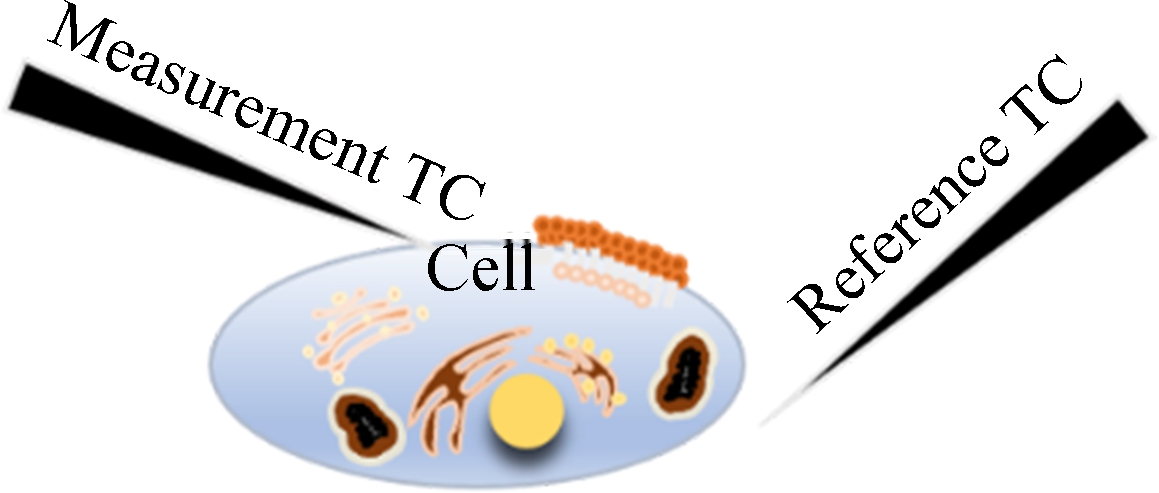
In order to further demonstrate the advantages of the dual-thermocouple difference method for the insensitivity to environmental disturbance, we prepared two experimental schemes. One was to use a single thermocouple for intracellular temperature measurement, and the other was to use a dual-thermocouple difference method for intracellular temperature measurement. Both experiments were conducted simultaneously in an open experimental environment, and changes in environmental temperature were recorded during the experiment. Fig.6 is a schematic diagram of the positions of thermocouples and cells.
(a)
(b)
Fig.6 Schematic diagram of two temperature measurement methods. (a) Single-thermocouple mode; (b) Dual-thermocouple mode
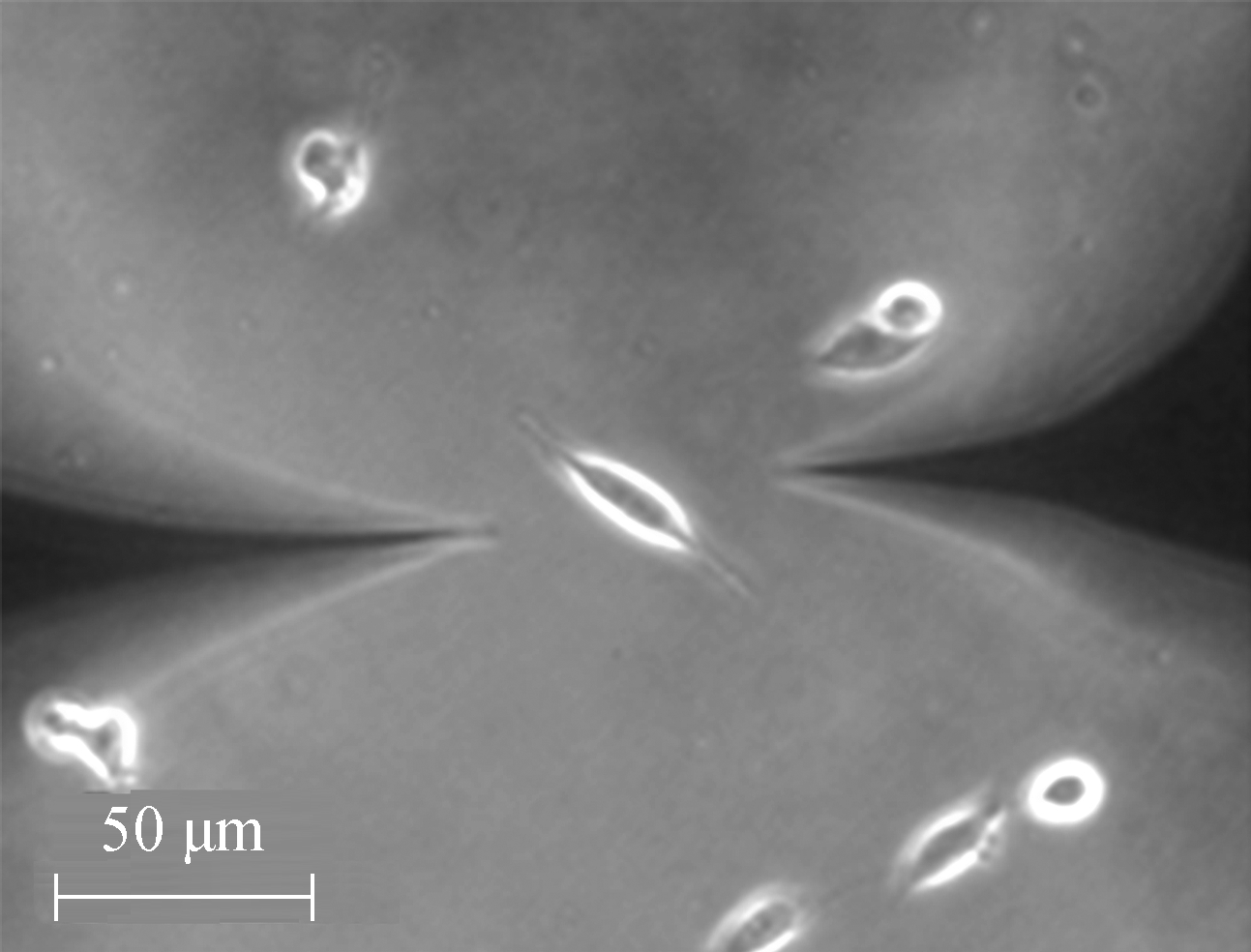
At the beginning of the temperature measurement, two TCs were carefully placed close to the cell under a normal culture and kept at 10 μm distance from the cell. As shown in Fig.7, the left probe is the measurement probe, and the right is the control probe. After the reading became stable, the left TC was moved to touch the cell and the right TC was kept in-situ.
(a)
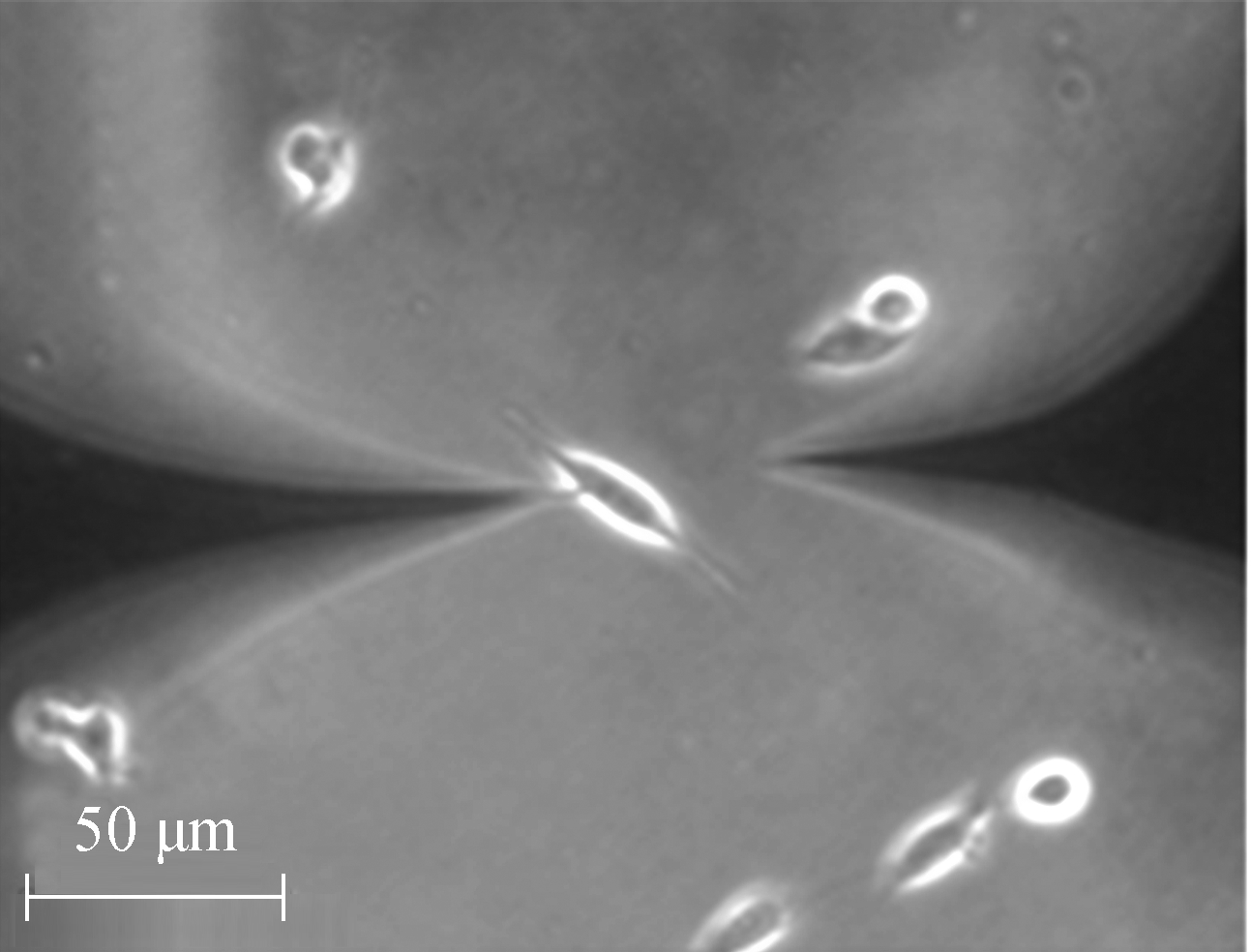
(b)Fig.7 Optical microscopic image of a living U251 cell. (a) Prior to the temperature measurement; (b) During the temperature measurement
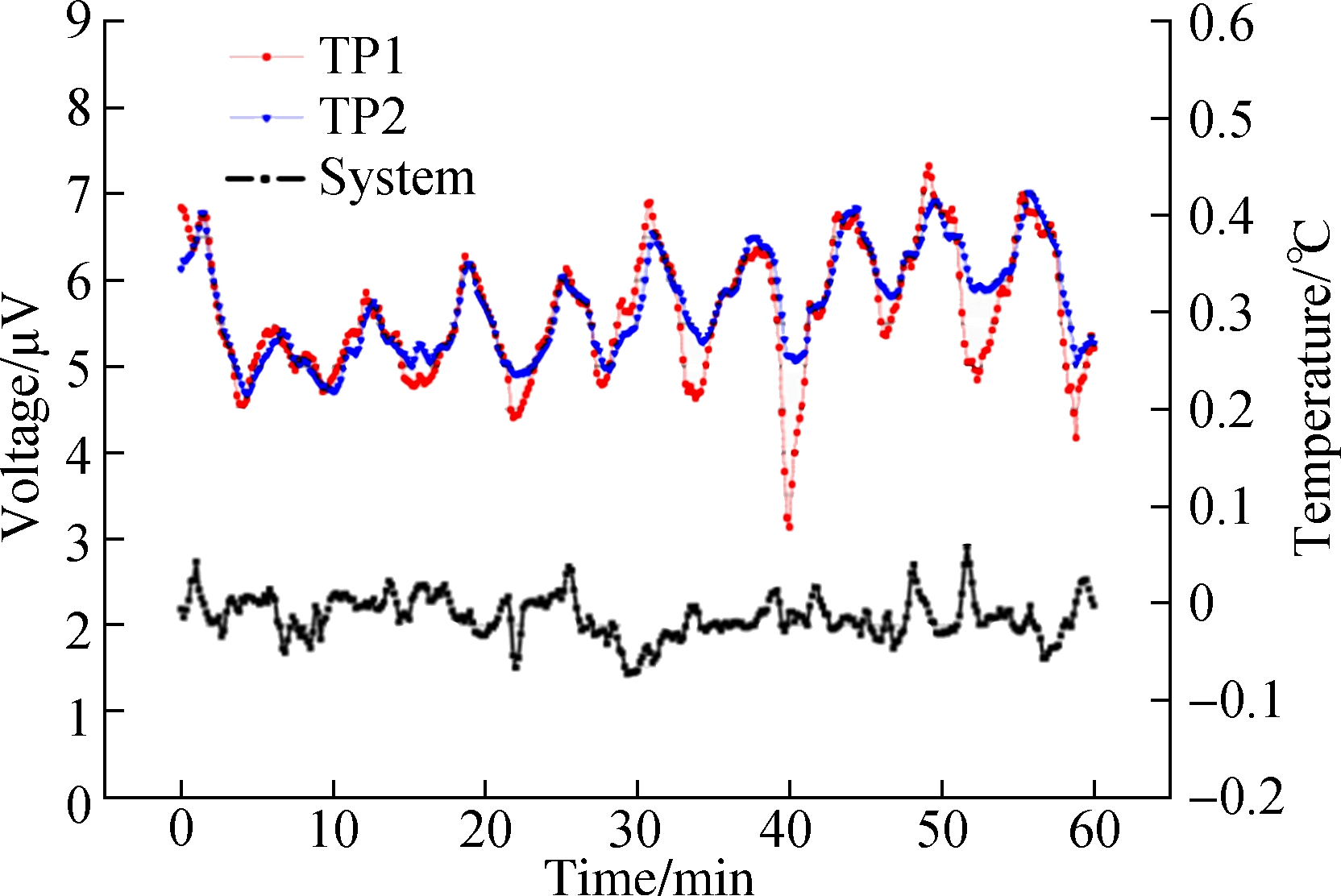
By monitoring the environmental temperature, we found that the open experimental environment would lead to temperature fluctuations. As shown in Fig.8(a), the circle indicated that the temperature fluctuation was significant. The three marked temperature values changed by about 0.2 ℃. We performed cellular temperature measurements in this environment, but the two methods showed different results. In the single thermocouple mode, the cellular temperature curve produced multiple peaks. The peaks marked by arrows correspond to temperature fluctuations in an environmental temperature image (see Fig.8(b)). The result indicated that the measurement of cellular temperature using a single thermocouple was susceptible to interference from the environmental temperatures. Fluctuations of 0.2 ℃ were easily confused with changes in cellular temperature. In the dual-thermocouple mode, there was only one peak in the cellular temperature curve and the fluctuation caused by environmental interference was weakened (see Fig.8(c)). Although both results showed a similar trend, the results obtained by the dual thermocouple difference method were smoother and more stable. It showed that the dual-thermocouple difference method was not sensitive to environmental interference. This method could effectively eliminate interference and improve the accuracy of cellular temperature measurement.

(a)

(b)
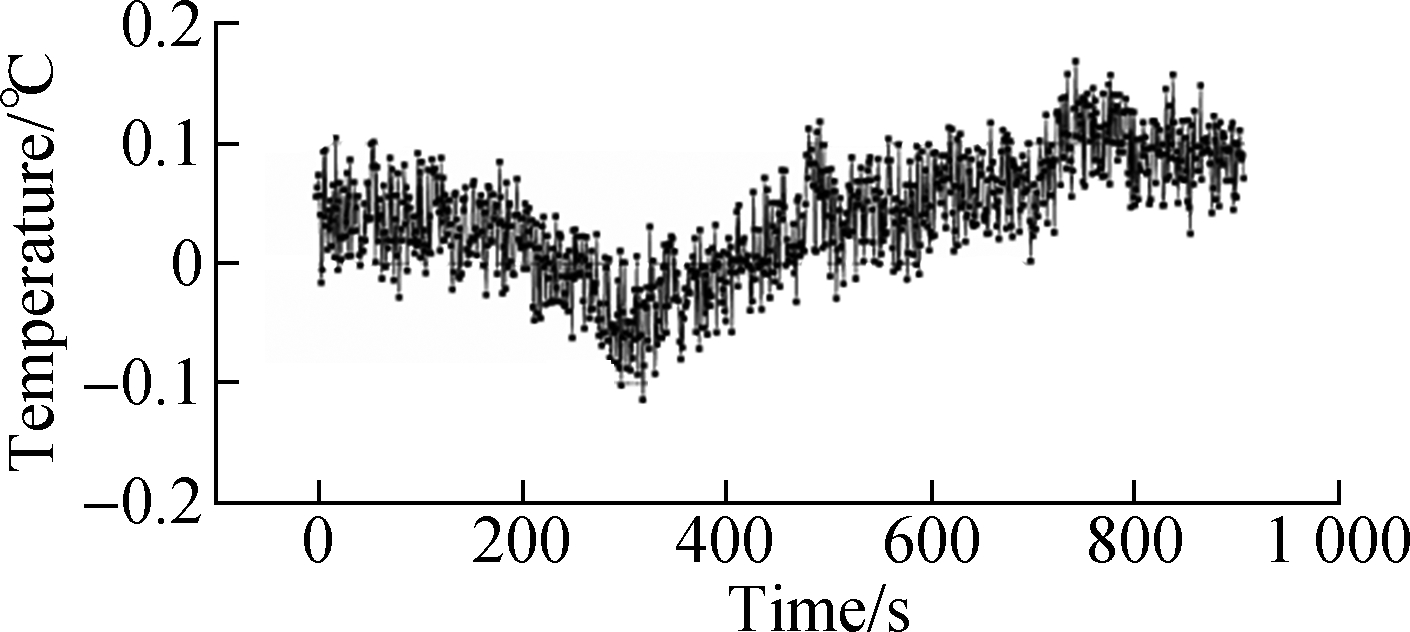
(c)Fig.8 The results of temperature measurement. (a) Temperature measurement of the experimental environment; (b) Cellular temperature measurement by a single thermocouple; (c) Cellular temperature measurement by a dual thermocouple
3 Conclusions
1) With the dual-thermocouple difference method, it was ensured that the obtained results could reflect temperature changes in single cell and not be disturbed by the environment.
2) Compared with single thermocouple, the dual-thermocouple mode not only can remove the setting of reference points, but also improve the anti-interference ability of the device.
3) The temperature measurement accuracy of this method was about 0.1℃, which could detect the tiny temperature fluctuations of a single cell.
4) The dual-thermocouple difference method was particularly suitable for evaluating the thermogenic ability of a single cell. The accurate and reliable measurement method will assist the study of intracellular thermodynamics and thereby help to explore the profound mechanisms of cells.
[1] Donner J S, Thompson S A, Kreuzer M P, et al. Mapping intracellular temperature using green fluorescent protein[J].Nano Letters, 2012, 12(4): 2107-2111. DOI:10.1021/nl300389y.
[2] Maksimov E G, Yaroshevich I A, Tsoraev G V, et al. A genetically encoded fluorescent temperature sensor derived from the photoactive orange carotenoid protein[J].Scientific Reports, 2019, 9: 8937. DOI:10.1038/s41598-019-45421-7.
[3] Kucsko G, Maurer P C, Yao N Y, et al. Nanometre-scale thermometry in a living cell[J].Nature, 2013, 500(7460): 54-58. DOI:10.1038/nature12373.
[4] Albers A E, Chan E M, McBride P M, et al. Dual-emitting quantum dot/quantum rod-based nanothermometers with enhanced response and sensitivity in live cells[J].Journal of the American Chemical Society, 2012, 134(23): 9565-9568. DOI:10.1021/ja302290e.
[5] Okabe K, Sakaguchi R, Shi B N, et al. Intracellular thermometry with fluorescent sensors for thermal biology[J].Pflügers Archiv—European Journal of Physiology, 2018, 470(5): 717-731. DOI:10.1007/s00424-018-2113-4.
[6] Suzuki M, Plakhotnik T. The challenge of intracellular temperature[J].Biophysical Reviews, 2020, 12(2): 593-600. DOI:10.1007/s12551-020-00683-8.
[7] Brites C D S, Lima P P, Silva N J O, et al. Thermometry at the nanoscale[J].Nanoscale, 2012, 4(16): 4799-4829. DOI:10.1039/c2nr30663h.
[8] Zhou J J, del Rosal B, Jaque D, et al. Advances and challenges for fluorescence nanothermometry[J].Nature Methods, 2020, 17(10): 967-980. DOI:10.1038/s41592-020-0957-y.
[9] Gota C, Okabe K, Funatsu T, et al. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry[J].Journal of the American Chemical Society, 2009, 131(8): 2766-2767. DOI:10.1021/ja807714j.
[10] Okabe K, Inada N, Gota C, et al. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy[J].Nature Communications, 2012, 3: 705. DOI:10.1038/ncomms1714.
[11] Suzuki M, Tseeb V, Oyama K, et al. Microscopic detection of thermogenesis in a single HeLa cell[J].Biophysical Journal, 2007, 92(6): L46-L48. DOI:10.1529/biophysj.106.098673.
[12] Wang C, Xu R, Tian W, et al. Determining intracellular temperature at single-cell level by a novel thermocouple method[J].Cell Research, 2011, 21(10): 1517-1519. DOI:10.1038/cr.2011.117.
[13] Tian W J, Wang C L, Wang J Q, et al. A high precision apparatus for intracellular thermal response at single-cell level[J].Nanotechnology, 2015, 26(35): 355501. DOI:10.1088/0957-4484/26/35/355501.
[14] Bai T T, Gu N. Micro/nanoscale thermometry for cellular thermal sensing[J].Small, 2016, 12(34): 4590-4610. DOI:10.1002/smll.201600665.
[15] Yang S, He W N, Li C, et al. A new approach of electrochemical etching fabrication based on drop-off-delay control[J].The Review of Scientific Instruments, 2019, 90(7): 074902. DOI:10.1063/1.5094470.
[16] Kallerhoff M, Karnebogen M, Singer D, et al. Microcalorimetric measurements carried out on isolated tumorous and nontumorous tissue samples from organs in the urogenital tract in comparison to histological and impulse-cytophotometric investigations[J].Urological Research, 1996, 24(2): 83-91. DOI:10.1007/BF00431084.
[17] Deberardinis R J, Lum J J, Hatzivassiliou G, et al. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation[J].Cell Metabolism, 2008, 7(1): 11-20. DOI:10.1016/j.cmet.2007.10.002.
[18] Inomata N, Van Toan N, Ono T. Liquid thermocouple using thermoelectric ionic liquids[J].IEEE Sensors Letters, 2019, 3(5): 1-4. DOI:10.1109/LSENS.2019.2912418.
[19] Yamamura M, Hayatsu H, Miyamae T. Heat production as a cell cycle monitoring parameter[J].Biochemical and Biophysical Research Communications, 1986, 140(1): 414-418. DOI:10.1016/0006-291X(86)91106-X.
[20] Lowell B B, Spiegelman B M. Towards a molecular understanding of adaptive thermogenesis[J].Nature, 2000, 404(6778): 652-660. DOI:10.1038/35007527.