Black cotton soil (BCS)has been found in abundance in Eastern Africa, and similar clays also abound in other places such as South Asia, Australia, and China[1]. Nairobi, the capital of Kenya, is located in the Robe Valley in Central Africa, where about 60% of the surface is covered by BCS. The soils are highly expansive when exposed to water during the rainy season[2-3]. However, the expansion seems to vanish during the following dry season[4-5]. The swelling pressure of BCS in Nairobi was reported to be 8-10 kg/cm2, and the volume change is as large as 120%-300%. These properties bring about serious problems in light construction on BCS. Iterative swelling-shrinkage and low bearing capacity always cause soil weakness and result in damage to highways such as slope failure, subgrade settlement, and pavement cracking. To find a strategy to overcome these problems for highway construction, the clay minerals in Nairobi BCS and their water swelling properties must be investigated.
Scientific mineral identification techniques have been developed in the last decade. Methods such as chemical analysis, X-ray diffraction (XRD) characterizations, differential thermal analysis, thermogravimetric analysis, infrared spectral absorption, and polarized light microscope plus microscopic analysis are commonly used[6]. Extensive research has been conducted on mineralogical identification of clay minerals by explaining diffraction data[7]. However, few reports on Nairobi BCS have been found in the field of mineralogical quantification for unknown reasons. The types of clay minerals in soils can be determined by comparing the XRD pattern with standard minerals. The potential for using XRD-based techniques for the quantification of clay minerals on the basis of identified phases has been investigated[8-9].
The swelling properties of clay minerals have been researched using experimentation and molecular simulation to investigate hydration, interlayer structure, and dynamics of interlayer positive ions in the clay[10-11]. The experimental techniques present a few limitations in exploring the microstructure. Molecular simulation has been regarded as a valuable tool for addressing unsolved issues related to clay swelling as a complement to experimental techniques. Molecular simulation directly employs structural information to investigate structure-function relationships. Nonequilibrium states related to expansive hysteresis may be studied systematically by using computational methods. Molecular simulation allows for research on sophisticated clay systems by using simplified conditions, e.g., the behavioral information of the individual interlayers can be acquired without complexities from the mixing layer hydrate formation or disturbance from outside surface effects.
In this research, experimental investigation and molecular simulation were revisited and used to identify the types of the clay minerals and explain the hydration of the interlayer irons to reveal the swelling properties of Nairobi BCS. The chemical constituent and XRD patterns were used to determine the types of the clay minerals and predict the percentages of various clay minerals. The adsorption of water molecules was simulated to analyze the combinative capabilities of the interlayer Na+, Ca2+, or Mg2+ ions with water. The hydration of the interlayer irons was also investigated to explain the strong expansion characteristics of Nairobi BCS. To confirm the classification standard of the expansive potential of BCS by using free swell index (FSI) as an indicator, the FSI test was performed and the correlation of FSI with the montmorillonite content and cation exchange capacity (CEC) of Nairobi BCS was investigated.
1 Materials and Experimental Methods
1.1 BCS sampling sites and pretreatments
BCS was sampled from a roadbed site in Nairobi, Kenya (36°31′E, 1°35′S), where a beltway called the Southern Bypass was being built by China Road and Bridge Co. The natural BCS is characterized by darkness and cracking, as shown in Fig.1. The samples were first cut into slices and air-dried for several days and then ground in a mortar until all soil particles passed through a 2.0 mm sieve. The soil particles with different sizes, such as clay (< 2 μm), silt (2-75 μm), and sand (75 μm-2.0 mm), were obtained in accordance with the standard method[12]. BCS consisted of 52% clay and 48% silt. The free swell ratio (FSR) of BCS was 165%, and the liquid limit and plasticity index were 57% and 26%, respectively, which indicates that BCS belongs to clay with high plasticity and expansive potential on the basis of JTG E40—2007[12].

Fig.1 Typical morphological characteristics of black cotton soil
1.2 Mineral phase analysis
The mineral phases were determined by XRD performed on BCS in accordance with the Chinese standard[13]. The BCS was first oven-dried until a constant weight was achieved at 60 ℃ and was ground in a mortar to make all soil particles less than 40 μm. The specimens were made by pouring the powders into a sample frame and pressing them in a vertical direction. The diffractograms were obtained by fixing the specimens on the holder of the XRD diffractometer (Ultima Ⅳ, CuKα radiation). Data were then recorded at a speed of 2(°)/min per step. X-ray fluorescence (XRF) was performed on a Thermo ADVANT’XP instrument to inspect the chemical composition of BCS.
1.3 FSI test
The FSI of BCS was determined in accordance with JTG E40—2007. The air-dried BCS was ground in a mortar until all soil particles passed through a 0.5 mm sieve opening. The soil samples were oven-dried at 105 ℃ until a constant weight was achieved then cooled down to room temperature in a dryer. Then, 10 g of soil was weighed and poured into a 50 mL measuring cylinder with 30 mL of distilled water. The mixture in the cylinder was stirred ten times from the liquid surface to the bottom of the cylinder by using a stirring rod. Then, the soil particles on the stirring rod and the inner surface of the cylinder were washed into the measuring cylinder until the liquid surface reached 50 mL. After standing for 24 h, the equilibrium sediment volume was noted. Distilled water was replaced with kerosene, and the same operational approaches were performed to obtain the equilibrium sediment volume of 10 g oven-dried soil passing a 0.5 mm sieve in kerosene. The FSR and the FSI can be calculated as
(1)
(2)
where δFSR is the free swell ratio of BCS; δFSI is the free swell index of BCS; V0 is the volume of the 10 g oven-dried soil; Vd is the equilibrium sediment volume of 10 g oven-dried soil passing a 0.5 mm sieve in distilled water; and Vk is the equilibrium sediment volume of 10 g oven-dried soil passing a 0.5 mm sieve in kerosene.
2 Molecular Mechanics Simulation
2.1 Potential energy model
The potential energy reflects the interatomic potentials between atoms or ions and can be simulated by the SPC/E model, which provides a good description of the balance, structure, and kinetic behavior of water. This model supposes every water molecule has three interactional potentials at the location of an atomic nucleus, the bond length of O-H is 0.1 nm, and the bond angle of H-O-H is 109.47°. Van der Waals force is described by the potential energy of Lennard-Jones (LJ) as follows:
(3)
where qi and qj are the charges on the atoms i and j, respectively; rij is the distance between atoms i and j; and εo is the dielectric constant. The LJ cross-interaction terms, εij and σij, are generated from the standard combining relationships,
(4)
(5)
2.2 Model construction
The model parameters come from Wyoming montmorillonite. The crystal texture of the montmorillonite is a clinorhombic system, and the space groups are C2/m. The octahedral coordination cations are located on the two sides of the symmetry plane. The cell parameters are as follows: a=0.523 nm, b=9.06 nm, α=γ=90°, β=99°. Value c is not a constant and ranges from 0.960 to 1.85 nm based on the types of the exchangeable cations and the numbers of water molecules among the structure layers. A supercell is composed of 8 unit cells in the form of 4a×2b×c, and water molecules were inserted into the interlayers of the supercell. The molecular formula of such montmorillonite supercell can be written as M0.75(H2O)n(Si7.75Al0.25)(Al3.5Mg0.5)O20(OH)4; M represents Ca2+, Mg2+, and Na+. The size of the supercell on XOY plane is 2.092 nm×1.812 nm. On the basis of Loewenstein law, one in 32 Si4+ was replaced by Al3+, and one in 8 Al3+ was replaced by Mg2+ in the supercell. The negative charges (-6 e) were compensated by the interlayer Na+, Ca2+, or Mg2+ ions after isomorphous substitution.
2.3 Simulation method
The materials science code Cerius2 was employed to compute the clay mineral hydration. Energy minimization and dynamics simulations were processed in the C2 Minimizer and C2 Dynamics modules, and the universal force field (UFF) in C2. The OFF module was chosen. The long-range electrostatic interaction was computed by Ewald summation. Van der Waals force was computed by the LJ function, and its cut-off radius was 0.9 nm. The charge was calculated by using the charge equilibration (QEq) method. After energy optimization by UFF, dynamic simulation was performed on the montmorillonite crystal lattice with one, two, and three layers of water molecules (32, 64, and 96 water molecules, respectively) under the microcanonical ensemble and 300 K.
3 Results and Discussions
3.1 Mineral phase identification
Tab.1 presents the chemical compositions of Nairobi BCS obtained by XRF and lists the literature data of the chemical composition of expansive soil in Ningming, China. BCS is characterized by a high iron (Fe2O3) content. The same characteristic was also found in India BCS. However, the iron content in Ningming soil is only 3.23%, which is far less than that in Nairobi BCS (9.44%). The literature previously established that the black color of the soils could be due to the presence of iron, manganese, and titanium in their reduced state. Nevertheless, the content of manganese and titanium in Nairobi BCS was only 0.3% and 0.9%, respectively. The high content of iron in its reduced state might be a principal factor in the dark color of Nairobi BCS. This finding is similar to previous findings.
Tab.1 Chemical (oxide) composition of BCS and Ningming expansive soil %

Oxide contentw(CaO)w(MgO)w(Fe2O3)w(Al2O3)w(SiO2)w(K2O)w(Na2O)w(SO3)w(P2O5)w(MnO)w(TiO2)LOIBCS1.700.959.4416.8950.341.010.760.150.020.300.9017.50Ningming soil0.201.453.2327.5955.453.070.160.170.020.337.35
Note:Data of Ningming soil were obtained from Ref.[14].
However,the darkness of clay is generally believed to be caused by the abundance of organic carbon. Therefore, a test was performed by pouring 1 g of Nairobi BCS into 20 mL of 30% hydrogen peroxide solution to remove the organic matter from the soil (see Fig.2). Organic matter in the soil is another factor that can contribute to the darkness of the BCS, as indicated by the comparison of the color change in 20 mL of 30% hydrogen peroxide solution and the water. Another noticeable characteristic of BCS is that the alkali content of CaO and Na2O is about 8.5 and 5 times that of Ningming soil, respectively, while the content of K2O is only about one-third that of Ningming soil. Hence, BCS is mainly composed of calcium- and sodium-based clay minerals, which presents higher expansibility than potassium-based clay minerals upon exposure to water.

Fig.2 1 g of BCS poured into 20 mL of 30% hydrogen peroxide solution (left) and distilled water (right)
Raw BCS particles on the glass slide were fixed on the X-ray measurements to primarily understand the mineral phases in BCS. Fig.3(a) presents the XRD pattern of the untreated BCS. The basic reflection peak is found at about d=1.579 05 nm, and its corresponding second- and multiple-harmonic peaks are obvious, thereby indicating the presence of periodical layered structures in BCS. The broader basic reflection peak also indicates the low crystallinity of those structures or the presence of mixed-layer minerals in BCS.

The clays were coated on a glass slide for XRD measurements to further confirm the presence of clay minerals in BCS after air drying, ethylene glycol saturation, and heating at 550 ℃. As shown in Fig.3(b), montmorillonite is detected by the reflection peak at d=1.448 54 nm in the N slide, which moves to d=1.816 75 nm in the pattern of clay saturated with ethylene glycol and collapses to d=1.009 07 nm after heat treatment. Ca-based smectite is always found at about 0.015 nm, but Na-based smectite is found at 0.012 5 nm; that is to say, Ca-based montmorillonite is the main clay mineral in BCS. Moreover, a small number of clay minerals such as interstratified illite-montmorillonite, illite, and kaolinite with other non-clay minerals such as quartz and feldspar are detected in Nairobi BCS. To calculate the relative amount of the clay minerals, overlapping peaks are resolved by using least-squares method, and curve fitting is performed by using the Lorentzian-Gaussian function. The proportion of montmorillonite, interstratified illite-montmorillonite, illite, and kaolinite is 32.6%, 10.9%, 2.3%, and 1.5%, respectively, according to the integration area. Moreover, a small amount of iron mineral phases such as goethite (0.6%) is determined in Nairobi BCS, which indicates that iron mainly exists in the form of clay minerals.
3.2 Hydration of interlayer cations
In general,the primary clay mineral in Nairobi BCS is montmorillonite with a small quantity of illite and kaolinite. Hence, the high expansive potential of Nairobi BCS mainly comes from the water swelling of montmorillonite. In addition, clays with different cation compositions and exchange capacities have a large difference in expansive potential. The CEC and exchangeable cations of Nairobi BCS and Ningming soil are presented in Tab.2. The CEC of Ningming soil is 106.87 meq/100 g and is larger than that of Nairobi BCS, which is 58.3 meq/100 g. However, the FSR and FSI of Ningming soil are only 93% and 104%, respectively, which are lower than the FSR and FSI of Nairobi BCS, which are 121% and 168%, respectively. Nairobi BCS has a high content of Mg2+ and Na+ with a percentage of up to 4.78% and 2.73%, respectively, and about 14 times that in the Ningming soil. These findings indicate that cation composition has a significant influence on the water absorption of montmorillonite. Hence, the montmorillonite crystal lattice with types of exchangeable cations in interlayers needs to be constructed to investigate the swelling properties of BCS.
Tab.2 CEC and exchangeable cations of BCS samples
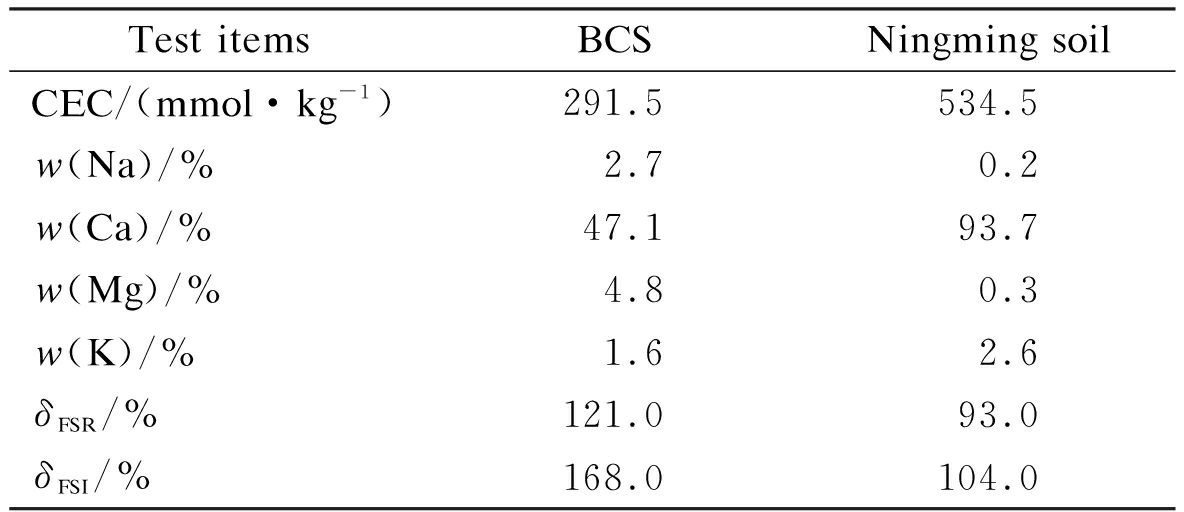
Test itemsBCSNingming soilCEC/(mmol·kg-1)291.5534.5w(Na)/%2.70.2w(Ca)/%47.193.7w(Mg)/%4.80.3w(K)/%1.62.6δFSR/%121.093.0δFSI/%168.0104.0
Note:Data of Ningming soil were obtained from Ref.[15].
Tab.3 presents the three montmorillonite structures, which are constructed to investigate the influence of Mg2+ and Na+ on the swelling properties of montmorillonite. The supercell of Type Ⅲ montmorillonite without water molecules in interlayers is shown in Fig.4. The pink octahedron represents the unsubstituted location of Al3+; the green octahedron represents the location of Al3+ replaced by Mg2+; the yellow tetrahedron represents the unsubstituted location of Si4+; the pink tetrahedron represents the location of Si4+ replaced by Al3+. The blue, green and purple spheres represent Ca2+, Mg2+, and Na+, respectively, which are used to balance the negative charges. The water molecule model is constructed on the Sketch atoms module and optimized on the Forceit module by using the SPC/E model and UFF.
Tab.3 Construction of three montmorillonite structures

ConfigurationCations and percentageCrystal textureⅠCa2+ (100%)Ca2+0.375(H20)n(Al3.5Mg0.5)(Si7.75Al0.25)O20(OH)4ⅡCa2+ (66.6%), Mg2+ (33.3%)Ca2+0.25 Mg2+0.125 (H20)n(Al3.5Mg0.5)(Si7.75Al0.25)O20(OH)4ⅢCa2+ (33.3%), Mg2+ (33.3%), Na+(33.3%)Ca2+0.125 Mg2+0.125 Na+0.125 (H20)n(Al3.5Mg0.5)(Si7.75Al0.25)O20(OH)4
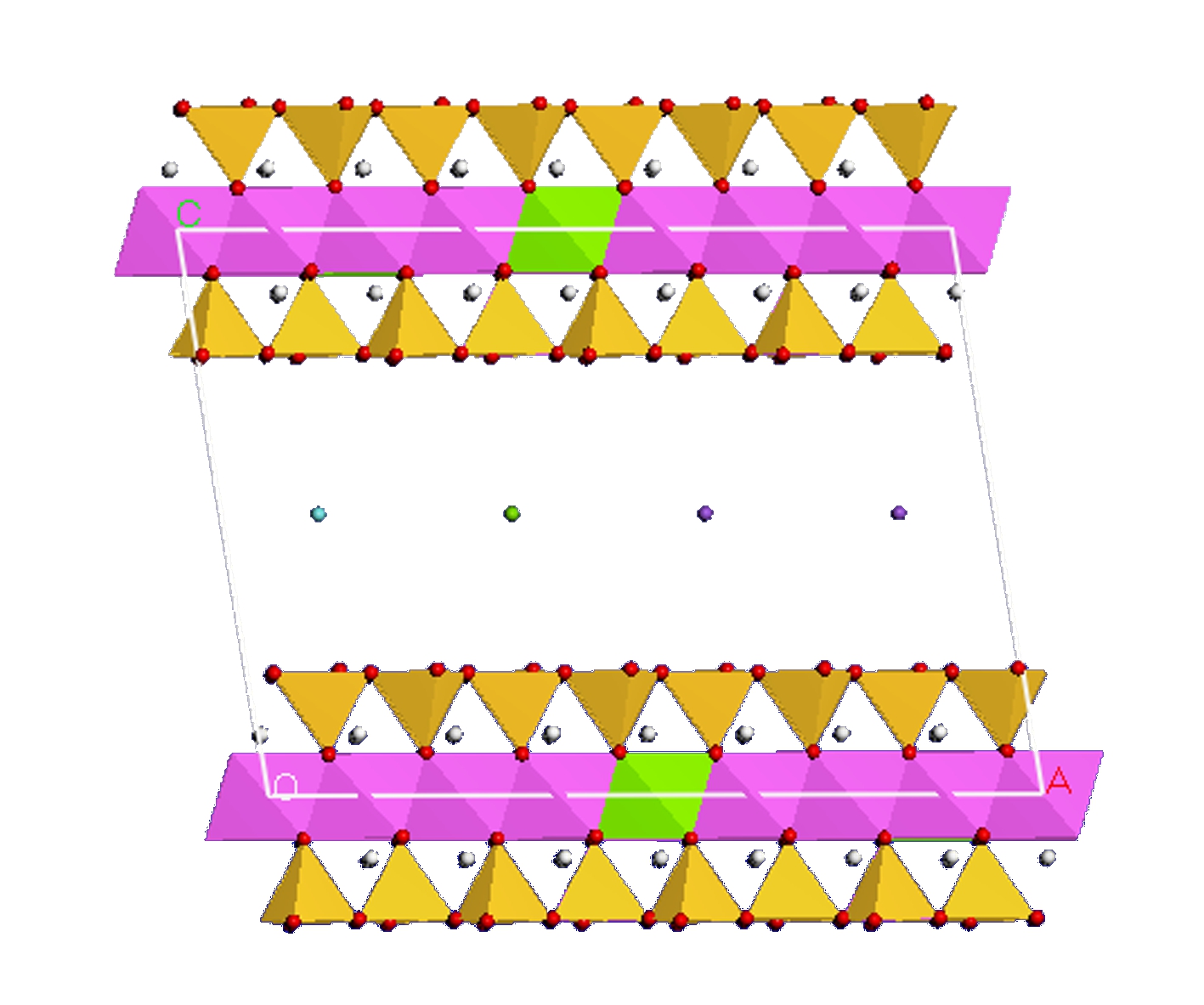
Fig.4 Supercell of Type Ⅲ montmorillonite without the water molecule
The adsorption capability for water molecules of three types of montmorillonite was simulated on the Sorption module. Type Ⅲ montmorillonite can adsorb 56 water molecules, which are larger than 51 and 53 water molecules of types Ⅰ and Ⅱ montmorillonite, respectively. A statistical analysis presented in Fig.5 was performed on the distribution of adsorption energy for the water molecules of those two types of montmorillonite to further confirm the adsorption capability for water molecules of types Ⅰ and Ⅲ montmorillonite. Type Ⅲ montmorillonite had a sharper distribution of adsorption energy, and the maximum and mean adsorption energy were 75 and 42 kJ/mol, respectively, but the values for Type Ⅰ montmorillonite were 63 and 38 kJ/mol, respectively. The analyses indicate that Type Ⅲ montmorillonite had stronger adsorption energy for water molecules and higher stability. The high expansive potential of Nairobi BCS comes from the strong adsorption capability for water molecules of montmorillonite with a high Mg2+ and Na+ content.

Fig.5 Adsorption energy distribution curve of water molecules for types Ⅰ and Ⅲ montmorillonite
The three types of montmorillonite with two layers of water molecules were investigated to compare the structural characteristics of the different types of montmorillonite with the same number of water molecules. If 1 kg of clay contains 0.1, 0.2, and 0.3 kg of water, then the distribution of water in montmorillonite is in the form of 1, 2, and 3 layers of water molecules, respectively, and the corresponding number of water molecules in the interlayer is 32, 64, and 96, respectively. The supercell of montmorillonite with two layers of water molecules can be obtained by inserting 64 water molecules into the interlayer. Fig.6 presents the supercell of Type Ⅲ montmorillonite with two-layer water molecules. After the structure optimization, molecular dynamics simulation was performed.
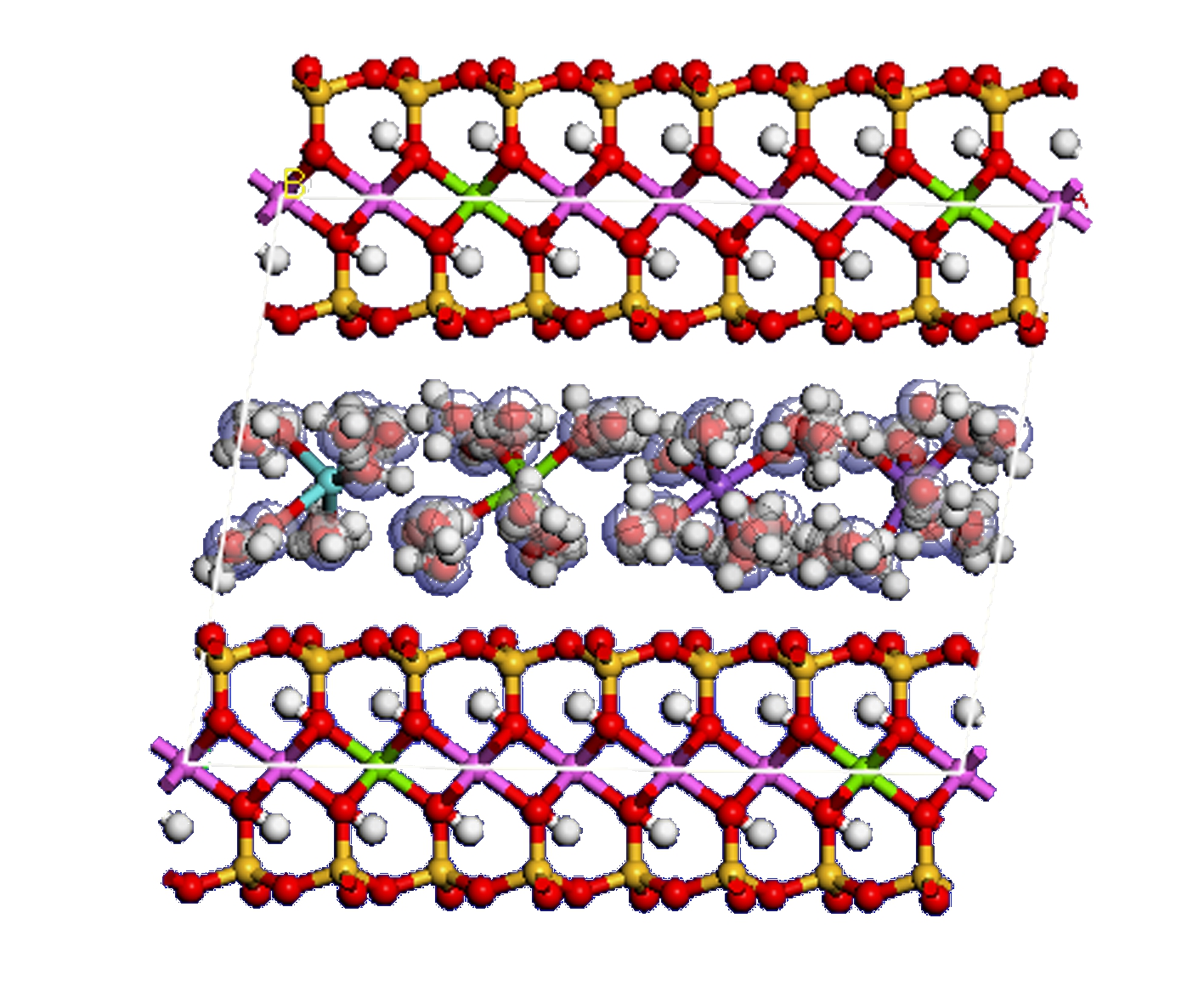
Fig.6 Supercell of Type Ⅲ montmorillonite with a two-layer water molecule
Fig.7 shows the radical distribution function (RDF) of Ca2+, Mg2+, and Na+ with Ow. The peak positions of Ca2+/Mg2+/Na+-Ow RDF in types Ⅰ, Ⅱ, and Ⅲ montmorillonite are shown in Tab.4. The first peak of Na-Ow, Mg-Ow, and Ca-Ow RDF occurs near 0.222 5 nm, 0.227 5-0.242 5 nm, and 0.237 5-0.262 5 nm in types Ⅰ, Ⅱ, and Ⅲ, montmorillonite, respectively. The difference of the first peak indicates that the positive ions have different capacities in organizing the oxygen atom. The Na-Ow RDF has a minimum first peak, which indicates that Na+ has the strongest interaction with the oxygen atom and is easy to hydrate, and is then followed by Mg2+ and Ca2+ in order. Moreover, the distance between Mg2+, Ca2+, and Ow reduces obviously in Type Ⅲ montmorillonite as compared with that in Type Ⅰ and Ⅱ montmorillonite. The small amount of Na+ facilitates the hydration of Mg2+ and Ca2+ to some extent. The major exchangeable cation in the clay minerals of Nairobi BCS is Ca2+ with some amount of Mg2+ and a few Na+. The Na+ content is too small to be the compensation ion but can facilitate the hydration of Mg2+, Ca2+. Hence, the clay minerals of Nairobi BCS have a high expansive potential.
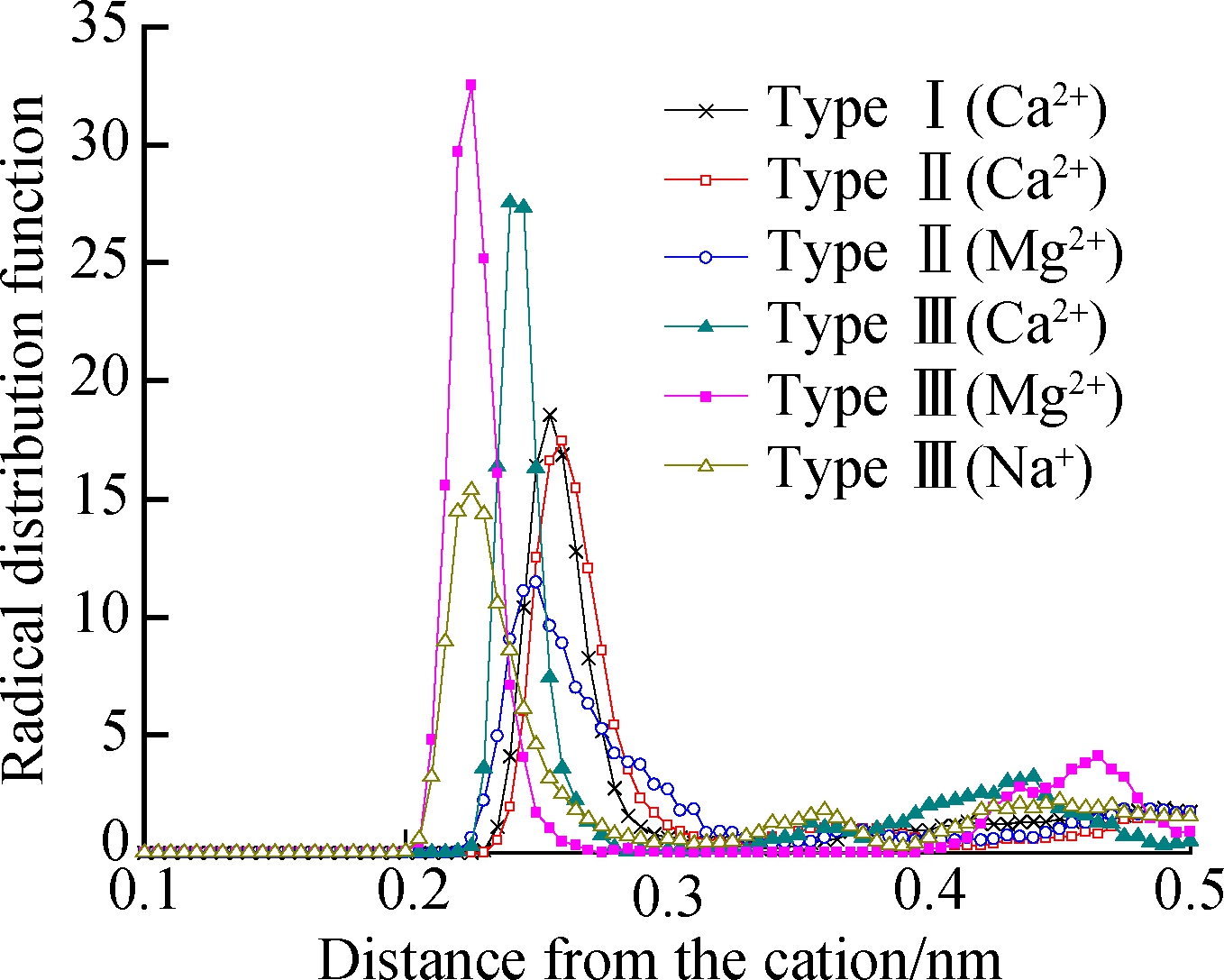
Fig.7 Graphs of the Ca2+/Mg2+/Na+-Ow RDF for montmorillonite hydrates
3.3 Identifying swell potential by FSI
The freeswell test of Nairobi BCS in the distilled water and kerosene was performed to estimate the rationalization of FSI as an index in determining the expansive potential of the clays. The expansive potential of the soils should be determined by the clay mineral content and the CEC because those two indexes can reflect the structural morphology and water adsorption capability, respectively. The FSI (FSR), CEC, and montmorillonite content were determined in the laboratory to verify the correlation of FSI with clay minerals and CEC.
The correlation of FSI and FSR with the montmorillonite content for Nairobi BCS is shown in Fig.8. In the figure, M represents the content of montmorillonite in clay. The FSI presents a good correlation with the montmorillonite content of Nairobi BCS, having a positive linear correlation coefficient of R2=0.968 1. However, the correlation of FSR with the montmorillonite content is relatively poorly qualified, and a positive linear correlation coefficient of R2=0.824 3 is obtained. The correlation of FSI and FSR with CEC(C) for Nairobi BCS is shown in Fig.9. A positive linear correlation coefficient of R2=0.941 6 means that FSI has a good correlation with CEC of Nairobi BCS, indicating better performance than FSR,which has a positive linear correlation coefficient of R2=0.852 3 with Nairobi BCS.
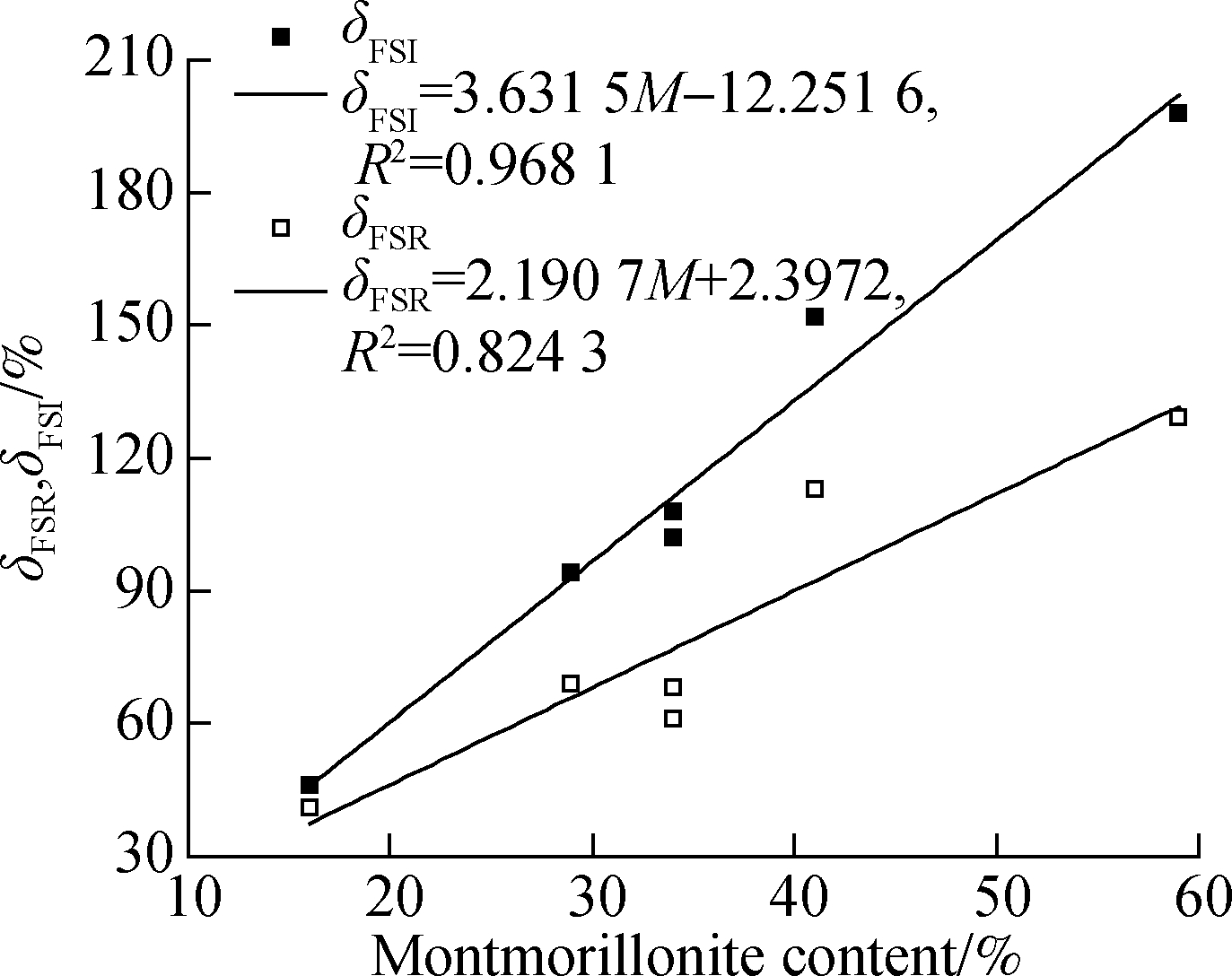
Fig.8 Correlation of FSR/FSI with montmorillonite content for Nairobi BCS
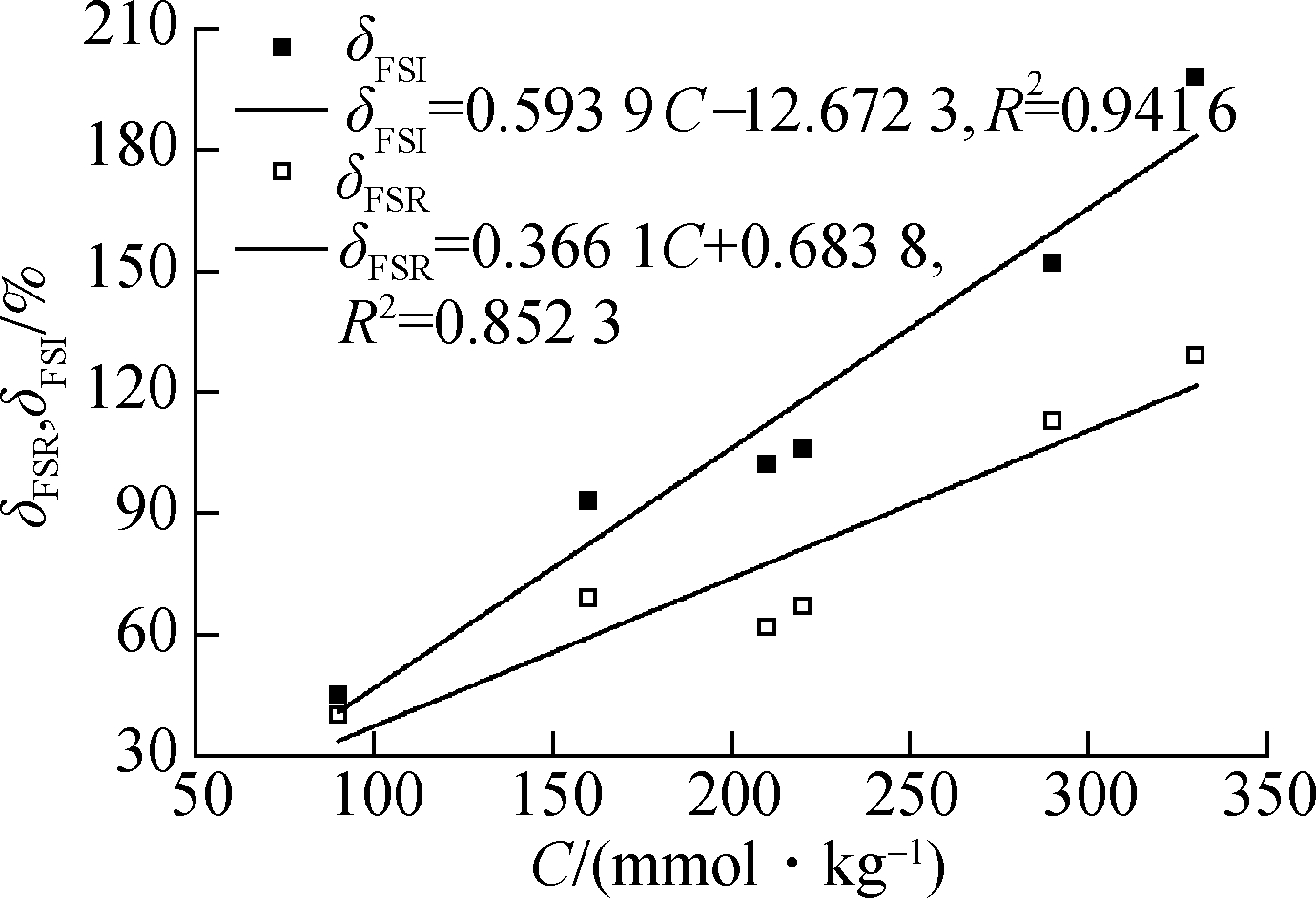
Fig.9 Correlation of FSR/FSI with CEC for Nairobi BCS
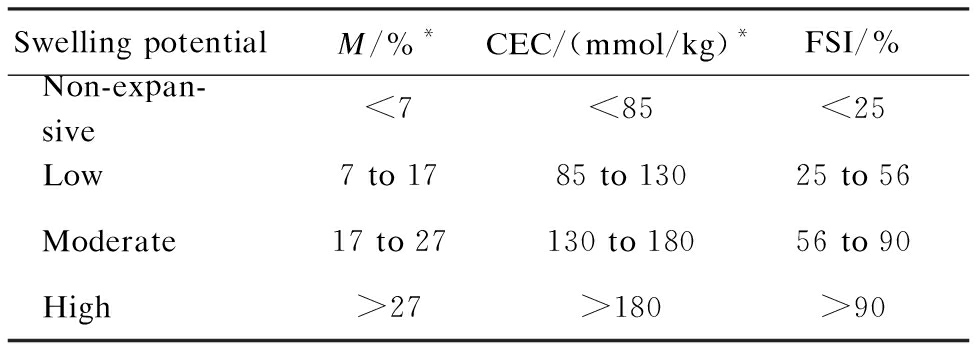
Overall, the FSI has a good linear correlation with intrinsic parameters that represent the swelling potential of the expansive soil for Nairobi BCS. Hence, FSI is ideally suited as a distinguishing index to determine the swelling potential of Nairobi BCS. Focused research has been conducted on the standards by using montmorillonite content and CEC as the classification indicators[16]. On the basis of the linear correlation of FSI with the montmorillonite content and CEC of Nairobi BCS, the criteria presented in Tab.4 can be obtained by using FSI as a classification indicator.
Tab.4 FSI criteria for identifying swelling potential
Swelling potentialM/%*CEC/(mmol/kg)*FSI/%Non-expan-sive<7<85<25Low7 to 1785 to 13025 to 56Moderate17 to 27130 to 18056 to 90High>27>180>90
Note:* Data were obtained from Ref.[16].
4 Conclusions
1) The high percentage of clay minerals, which are composed of montmorillonite (32.6%), interstratified illite-montmorillonite (10.9), illite (2.3%), and kaolinite (1.5%), is the major reason for the high expansive potential of Nairobi BCS.
2) The black color of Nairobi BCS is due to the presence of iron in its reduced state and organic carbon.
3) A limited number of Na+ in BCS enhances the adsorption capacity for water molecules of montmorillonite and accelerates the hydration of Mg2+, Ca2+ in clays, which ultimately leads to the high expansion of Nairobi BCS.
4) FSI presents a good linear correlation with the montmorillonite content and CEC, and it can be used as an index to classify the swelling potential of BCS.
[1]Patel S. Engineering properties of black cotton soil-dolime mix for its use as subbase material in pavements[J]. International Journal of Geomate, 2015, 8(1): 1159-1166. DOI:10.21660/2015.15.4191.
[2]Zhang P P, Huang J Q, Shen Z P, et al. Fired hollow clay bricks manufactured from black cotton soils and natural pozzolans in Kenya[J]. Construction and Building Materials, 2017, 141: 435-441. DOI:10.1016/j.conbuildmat.2017.03.018.
[3]Noolu V, Mudavath H, Pillai R J, et al. Permanent deformation behaviour of black cotton soil treated with calcium carbide residue[J]. Construction and Building Materials, 2019, 223: 441-449. DOI:10.1016/j.conbuildmat.2019.07.010.
[4]Gilbert B,Comolli L R, Tinnacher R M, et al. Formation and restacking of disordered smectite osmotic hydrates[J]. Clays and Clay Minerals, 2015, 63(6): 432-442. DOI:10.1346/ccmn.2015.0630602.
[5]Cheng Y Z, Huang X M, Li C, et al. Soil-atmosphere interaction as triggering factors of openings between embankment and pavement[J].KSCE Journal of Civil Engineering, 2018, 22(5): 1642-1650. DOI:10.1007/s12205-017-0679-6.
[6]Nkalih Mefire A, Njoya A, Yongue Fouateu R, et al. Occurrences of Kaolin in Koutaba (west Cameroon): Mineralogical and physicochemical characterization for use in ceramic products[J]. Clay Minerals, 2015, 50(5): 593-606. DOI:10.1180/claymin.2015.050.5.04.
[7]Zhou C H, Zhao L Z, Wang A Q, et al. Current fundamental and applied research into clay minerals in China[J].Applied Clay Science, 2016, 119: 3-7. DOI:10.1016/j.clay.2015.07.043.
[8]Ammar M, Oueslati W, Ben Rhaiem H, et al. Quantitative XRD analysis of the dehydration-hydration performance of (Na+, Cs+) exchanged smectite[J]. Desalination and Water Treatment, 2014, 52(22/23/24): 4314-4333. DOI:10.1080/19443994.2013.803324.
[9]Jozanikohan G, Sahabi F, Norouzi G H, et al. Quantitative analysis of the clay minerals in the Shurijeh Reservoir Formation using combined X-ray analytical techniques[J]. Russian Geology and Geophysics, 2016, 57(7): 1048-1063. DOI:10.1016/j.rgg.2016.06.005.
[10]Zheng Y, Zaoui A. Temperature effects on the diffusion of water and monovalent counterions in the hydrated montmorillonite[J]. Physica A: Statistical Mechanics and Its Applications, 2013, 392(23): 5994-6001. DOI:10.1016/j.physa.2013.07.019.
[11]Ngouana W B F, Kalinichev A G. Structural arrangements of isomorphic substitutions in smectites: Molecular simulation of the swelling properties, interlayer structure, and dynamics of hydrated Cs-montmorillonite revisited with new clay models[J]. The Journal of Physical Chemistry C, 2014, 118(24): 12758-12773. DOI:10.1021/jp500538z.
[12]Research Institute of Highway Ministry of Transport. Test methods of soils for highway engineering: JTG E40—2007 [S]. Beijing: China Communications Press, 2007. (in Chinese)
[13]National Energy Administration. Analysis method for clay minerals and ordinary non-clay minerals in sedimentary rocks by X-ray diffraction: SY/T 5163—2010[S]. Beijing: Petroleum Industry Press, 2010. (in Chinese)
[14]Li S L, Bo Z Z, Qin S J, et al. Application of plasitcity chart in distinguishing swelling soil[J]. Geological Review, 1984, 30(4): 352-356. DOI: 10.3321/j.issn:0371-5736.1984.04.007. (in Chinese)
[15]Tang H. Research on swelling characteristics and treatment technology of black cotton soil in East Africa[D]. Nanjing: Southeast University, 2017. (in Chinese)
[16]China Railway First Survey and Design Institute Group Co., Ltd. Code for special soil and rock investigation of railway engineering: TB 10038—2012 [S]. Beijing: China Railway Publishing House Co., Ltd., 2012. (in Chinese)