Incineration of solid waste has a risk of heavy metal emission to the atmosphere because some semi-volatile metals, such as lead and cadmium, can escape from the dust precipitator carried by ultrafine particulates[1-2].These metals vaporize as gaseous phase at a high temperature and then condense homogeneously and heterogeneously into a solid phase when flue gas is cooled[3-6].Moreover, municipal solid waste often contains a great amount of chlorine from food waste(NaCl)and plastic(PVC), which enhances the volatility of metals and intensifies their emission because lead and cadmium tend to transform to their chlorides at low melting points with the presence of chlorine[7-8].
Based on their transference natures, the in-situ adsorption in the furnace by minerals has been demonstrated as an effective treatment for combustion pollutions caused by semi-volatile metals[9].Layered silicates, including kaolinite, montmorillonite, and bentonite, have been successfully adopted to solve this issue as they can adsorb gaseous metals, such as K, Na, Pb, Cd, and Zn, and fix them via eutectic melting[10-12].Among these minerals, kaolinite is one of the most popular sorbents owing to its favorable ability to control particulate matter emission induced by semi-volatile metals[13-15] and considerable performance on heavy metal adsorption[16-18].Furthermore, its adsorptions of metal chlorides are more difficult than those of oxides, and due to the high formation probability of heavy metal chlorides during waste incineration, the improvement of chloride adsorption by silicates has attracted researchers’ attention[19-21].
Besides these silicates, some calcium-based sorbents, such as lime(or limestone, converted to lime at a high temperature), also show adsorption ability on heavy metals[22-23].Reactions between lime and gaseous metal chlorides(600 to 900 ℃)have been demonstrated[24], but their equilibrium constants rapidly drop down with temperature increase, so the adsorption ability of lime for metal chlorides is reduced at high temperatures[25-26].
Several studies have investigated the effect of the combined calcium—kaolinite in-furnace sorbent, one purpose of which was to utilize their different compounds for the control of multiple gaseous pollutants.For example, kaolinite can capture semi-volatile metals, such as Na, K, Pb, and Cd, to prevent them from transforming to or condensing onto ultrafine particles, and calcium can adsorb Hg and acid gases, such as SO2 and As2O3, which also have high toxicity[17,27].The interaction between calcium and kaolinite can generate glassy minerals with low melting points that enhance the adsorption reaction through the eutectic effect and liquid-gas capture, but the sintering effect destroys the sorbent structure and decreases its surface area, resulting in deactivation[28].Our recent work has reported that the occurrence of calcium might competitively occupy the reactive sites of aluminum silicates to inhibit the fixing of heavy metals, and we also found that in some conditions, the fixation of zinc could be enhanced by calcium doping[29-30].In summary, according to previous studies, inhibition and enhancement impacts on metal fixation were found when blending calcium and aluminum silicates during combustion, and kaolinite and limestone are also common additives and minerals in solid fuels, including coal and sludge, which often co-combust with other waste.However, the effect mechanisms of these mineral reactions have not been deeply investigated[30-33].In addition, limestone has a greater ability to capture cadmium than lead, whereas that for kaolinite is the opposite[11].Hence, can the mixture of the two minerals perform well in capturing both metals in a furnace?
Accordingly, this work studies the effect of mixed calcium for heavy metal capture by kaolinite in a furnace.A two-stage fixed-bed reactor was set up for the adsorption tests, and PbCl2 and CdCl2 were selected as sorbates.Besides adsorption efficiency, the characteristics of adsorption products were analyzed, including their morphologies and crystal phases.Moreover, the forms of heavy metal captured by the sorbents were classified to reveal the sorption process.
1 Materials and Experimental Methods
1.1 Materials
The sorbates were PbCl2 and CdCl2, both in powder with a purity of 99.9%.The main components of kaolinite were SiO2(42.8%), Al2O3(40.8%), and H2O(14.2%), and that of limestone was CaCO3(99.0%).Initially, both minerals were present in powder, and two kinds of mixed sorbents were made by blending kaolinite and limestone in some fractions and then stirring.The compositions of the sorbents are listed in Tab.1.
Tab.1 Compositions of sorbents
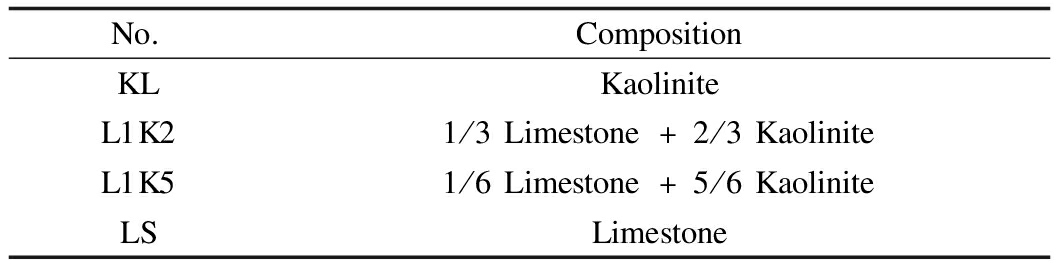
No.CompositionKLKaoliniteL1K21/3Limestone+2/3KaoliniteL1K51/6Limestone+5/6KaoliniteLSLimestone
To decrease the influence of heat transfer, each kind of sorbent was compressed into a tablet, broken, and sieved into particles of 0.2 to 0.3 mm diameter.
1.2 Vaporization and adsorption of heavy metal chlorides
A lab-scale horizontal tube furnace with two heating zones was used for the adsorption experiments, where both heating zones could be controlled independently by two temperature controllers.The adsorption temperatures were set at 700, 800, and 900 ℃.Metal chlorides with a weight of 0.025 g were loaded in the upper chamber for vaporization and carried by the N2 flow to the lower chamber to load the sorbent.The gas stream with the gaseous heavy metal passed over the sorbent layer for sorption, and the product was collected after the reaction for further analysis.More details of the reaction and experimental procedures can be found in our previous work[26,34-35].
1.3 Analysis methods
To identify the species of adsorbed heavy metals, adsorption products were digested by two methods.First, the samples were leached in adequate aqua regia under stirring at 25 ℃ for 2 h to obtain the leached form of the heavy metals, which could be regarded as the fraction of metal on the sorbent surface or combined with the aluminum oxides.Secondly, the products were digested thoroughly by HCl-HNO3-HF to obtain the total amount of the metals.An atomic absorption spectrometry was used to measure the concentrations of the two metals in the two solutions, and the partitions of the metal forms were calculated as follows:
(1)
(2)
Fr=Ef-Fl
(3)
where m0, ml, and ma are the mass of the element input(calculated by 0.025 g PbCl2 and CdCl2)and masses of the metal in the leachate liquid and total digestion solution, respectively; Fl, Fr, and Ef are the fractions of the leached and residual form in the adsorption product and the adsorption efficiency, respectively.For each condition, three parallel tests were conducted, and their average values were used for data analysis.
X-ray diffraction(XRD)was used to detect the crystal phases of the adsorption products, and their morphologies were observed via scanning electron microscopy and electronic disperse spectrometry(SEM-EDS).
2 Results and Discussions
2.1 Mineral phases and morphologies of the adsorption products
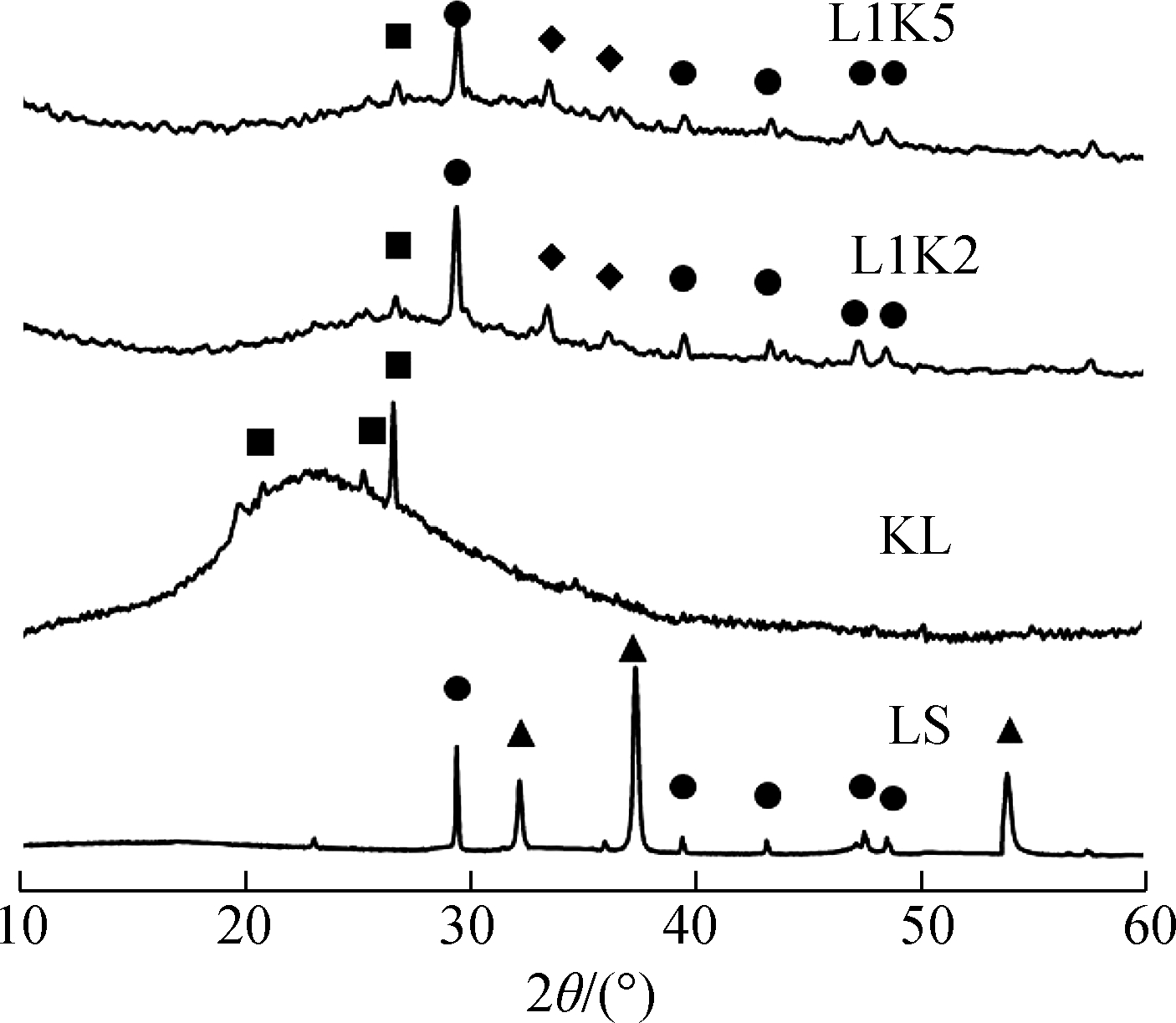
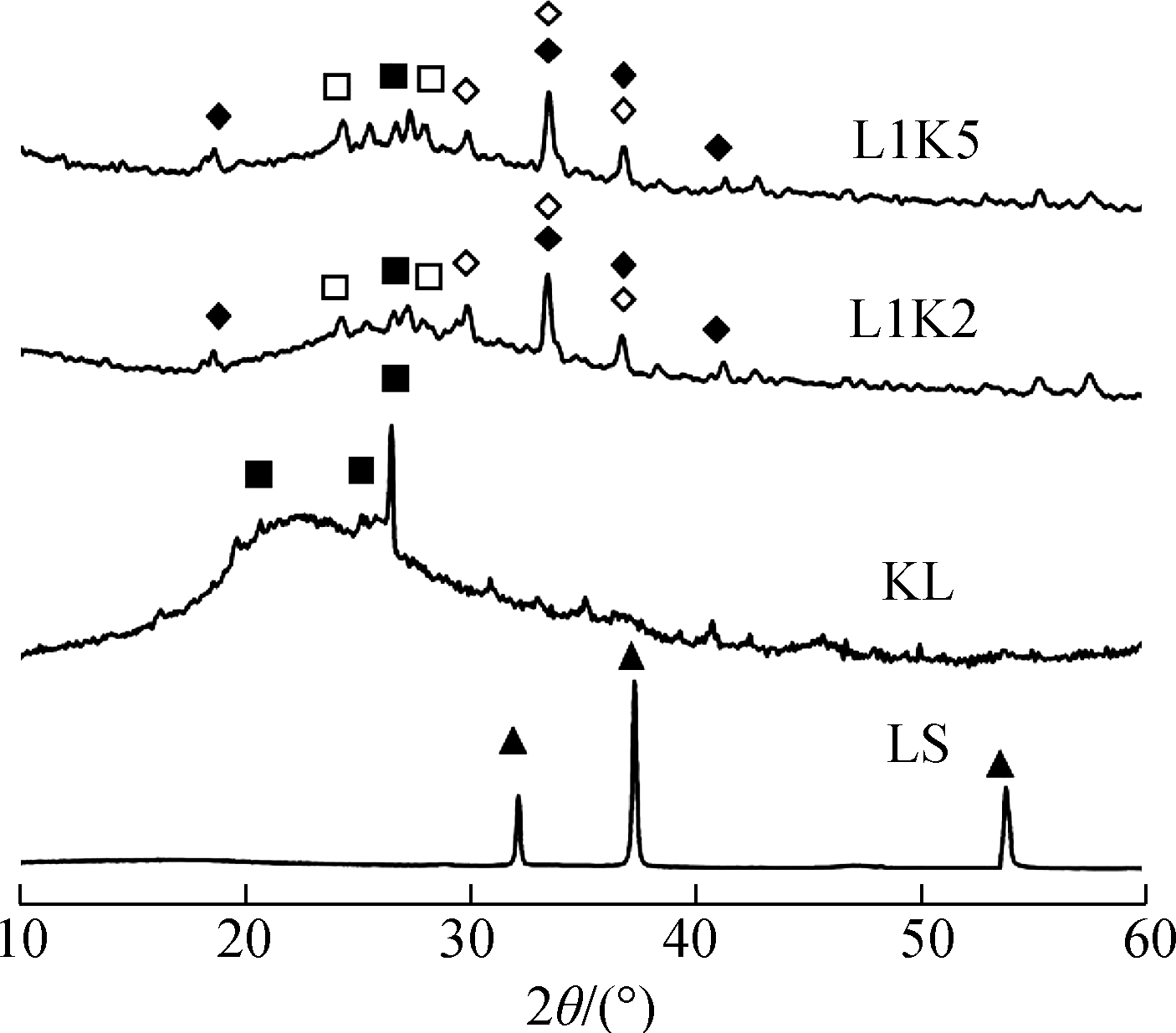
The crystal phases in the sorbent after reactions are shown in Fig.1.Because of the low quantities of input sorbates, no crystal with heavy metal was detected from the XRD analysis, and it had little effect on the dominant minerals of Ca-Si-Al.The mineral phase changes were discussed only within the Ca-Si-Al system.
(a)
(b)

(c)
![]() SiO2 ;
SiO2 ;  CaCO3;
CaCO3;  Ca12Al14O33;
Ca12Al14O33;  Ca3Al2Si3O12;
Ca3Al2Si3O12;  CaO
CaO![]() CaAl2Si2O8;
CaAl2Si2O8;  Ca2Al2SiO7;
Ca2Al2SiO7;  Al6Si2O13
Al6Si2O13
Fig.1 XRD curves and detected crystal phases in the adsorption products.(a)700 ℃;(b)800 ℃;(c)900 ℃
A part of limestone(calcite phase)was decomposed into lime at 700 ℃ and then transformed completely after 800 ℃.For the products of kaolinite, there was only amorphous phase and quartz, and mullite was found at 900 ℃.
More kinds of minerals were found in the products of the mixed sorbents.At 700 ℃, mayenite was generated, which might be due to the reaction between calcium and activated aluminum from metakaolinite.Calcite was still detected in products of both mixed sorbents, and no peak of lime was found, indicating the lower reactivity of calcite than that of lime, and all limes reacted with aluminum silicates.Anorthite and grossular appeared at 800 ℃, and calcite disappeared at the same time.Then, at 900 ℃, quartz was thoroughly consumed to react with calcium minerals, and gehlenite was newly found in the XRD curves.It should be noted that anorthite is the ultimate product of the kaolinite—calcium reaction[35], but in this case, there were heterogeneous reactions between silicates and calcium in the mixed sorbents, where the generation and crystallization of Ca-Si-Al minerals were promoted at high temperatures, indicating a further interaction between calcium and silicates.
Overall, both products of the mixed sorbents shared the same species of crystal, which means a mineral found in a product of a calcium-mixed sorbent could be detected in another mixed sorbent product reacted at the same temperature.The difference was that the peaks of the calcium mineral of L1K2 were stronger than those of L1K5 because of the higher percentage of blended calcium.Consequently, under the experimental conditions of this work, the calcium fraction did not decide the species of calcium crystals but affected the quantity of the minerals.However, amorphous silicates cannot be recognized by XRD, which could also be generated at a high temperature[30,35].
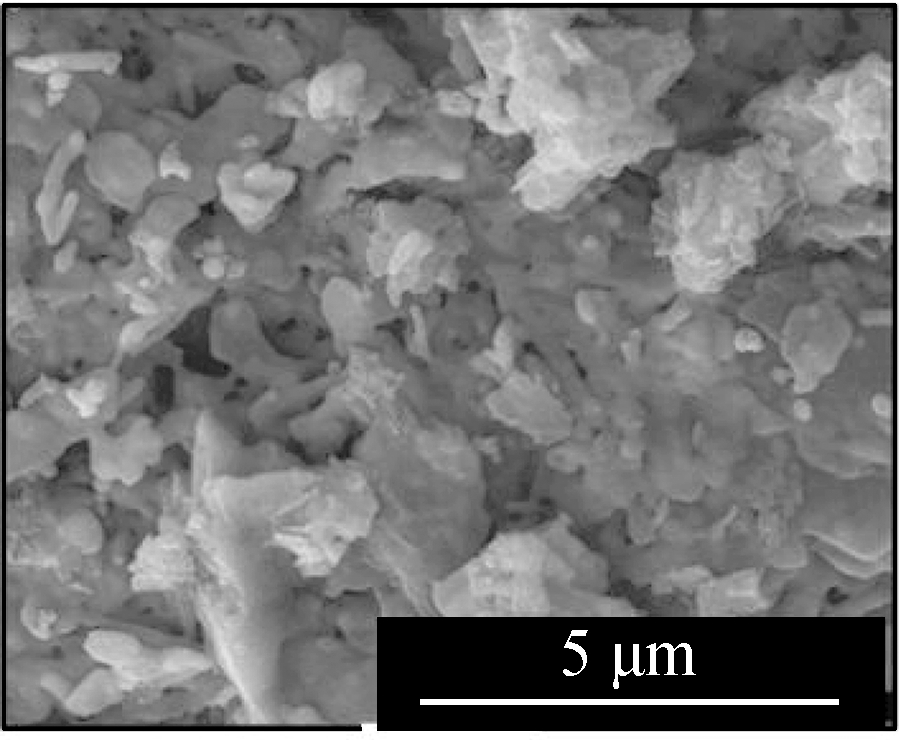
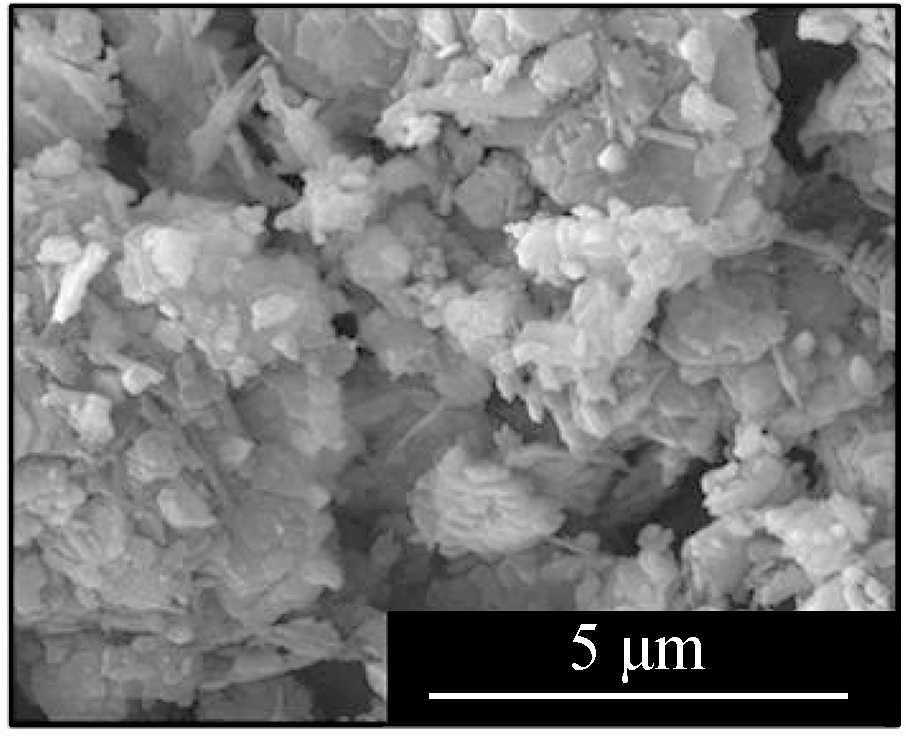
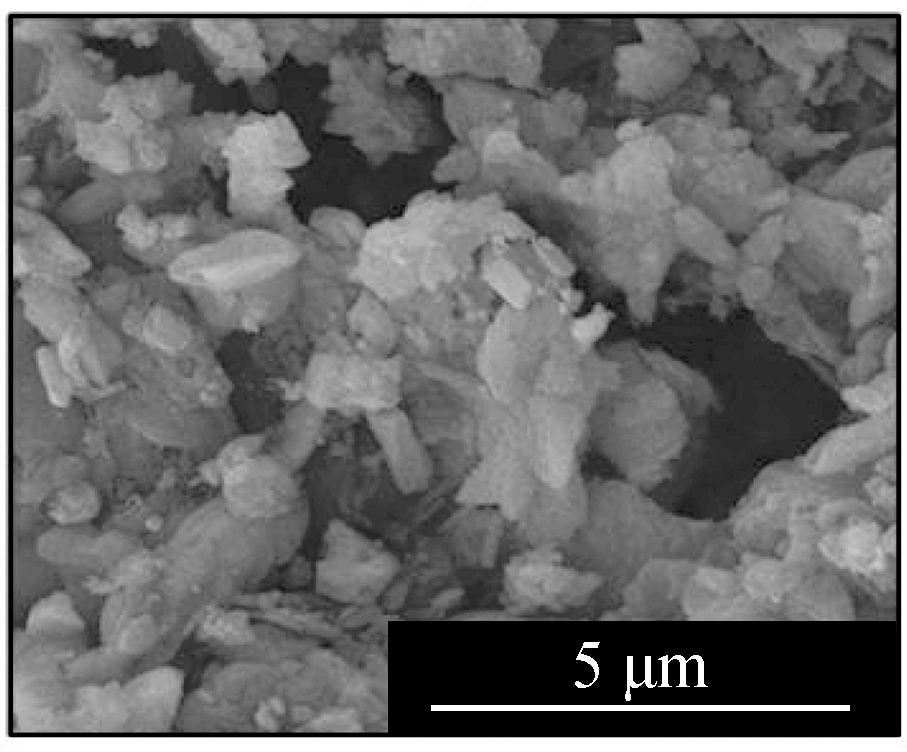
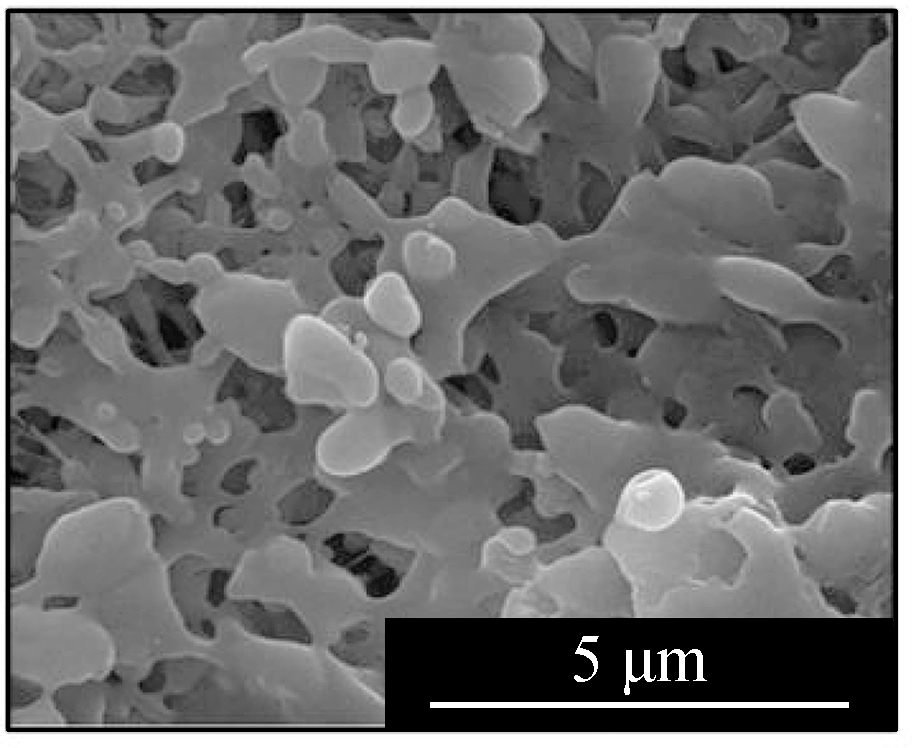
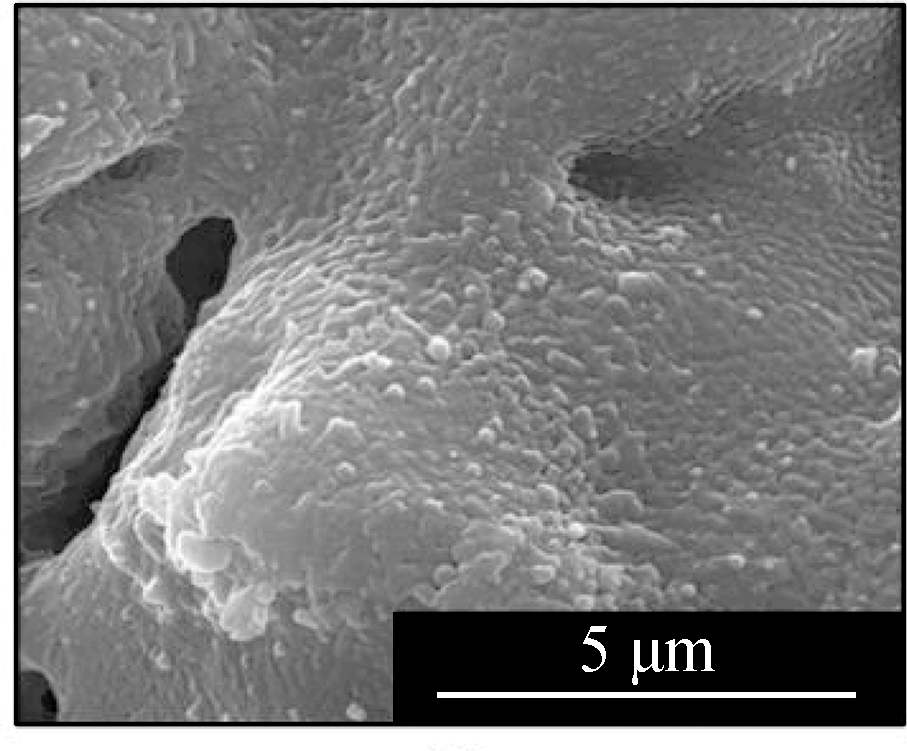
Fig.2 displays the morphologies of the adsorption products by the mixed sorbents, of which the elemental analysis is listed in Tab.2.The overall sample of L1K2 at 700 ℃(see Fig.2(a))shows a slight sinter, whereas the typical layered structure of kaolinite was still clear, but that at 900 ℃(see Fig.2(d))was an almost molten surface.For the samples of L1K5, two kinds of morphologies were found, of which ones were similar to those of L1K2 when comparing Fig.2(a)with Fig 2(c)and Fig.2(d)with Fig.2(e).The EDS analysis also shows the areas rich in calcium.However, areas in Fig.2(b)and(f)contained far less calcium and seem to maintain a layered structure because of the few fractions of calcium blending.The reason for the phenomena was that calcium enhanced the molten effect to form low-melting-point silicates, and its impact became greater with the temperature increase.Here the Ca-rich zone has an atomic ratio of(Si+Al):Ca is smaller than 2, whereas for the Ca-low zone; the ratio is bigger than 2.Therefore, because the sample of L1K2 shows only one single phase and that of L1K5 presents Ca-rich and Ca-low areas at 900 ℃, the sorbent of L1K2 can be regarded as Ca saturated, whereas L1K5 is calcium unsaturated, which could lead to different characteristics in their adsorption performance.
Tab.2 Atomic percentages of the displayed areas %

DisplayedareaSiAlCaCClPbFig.2 a 141219621Fig.2 b 1820612<1Fig.2 c 14131842<1Fig.2 d 171525011Fig.2 e 14171801<1Fig.2 f 2621100<1<1
(a)
(b)
(c)

(d)
(e)
(f)
Fig.2 SEM images of the adsorption products.(a)L1K2 at 700 ℃;(b)L1K5 at 700 ℃, layered surface;(c)L1K5 at 700 ℃, molten surface;(d)L1K2 at 900 ℃;(e)L1K5 at 900 ℃, molten surface;(f)L1K5 at 900 ℃, layered surface
2.2 Distributions of heavy metals
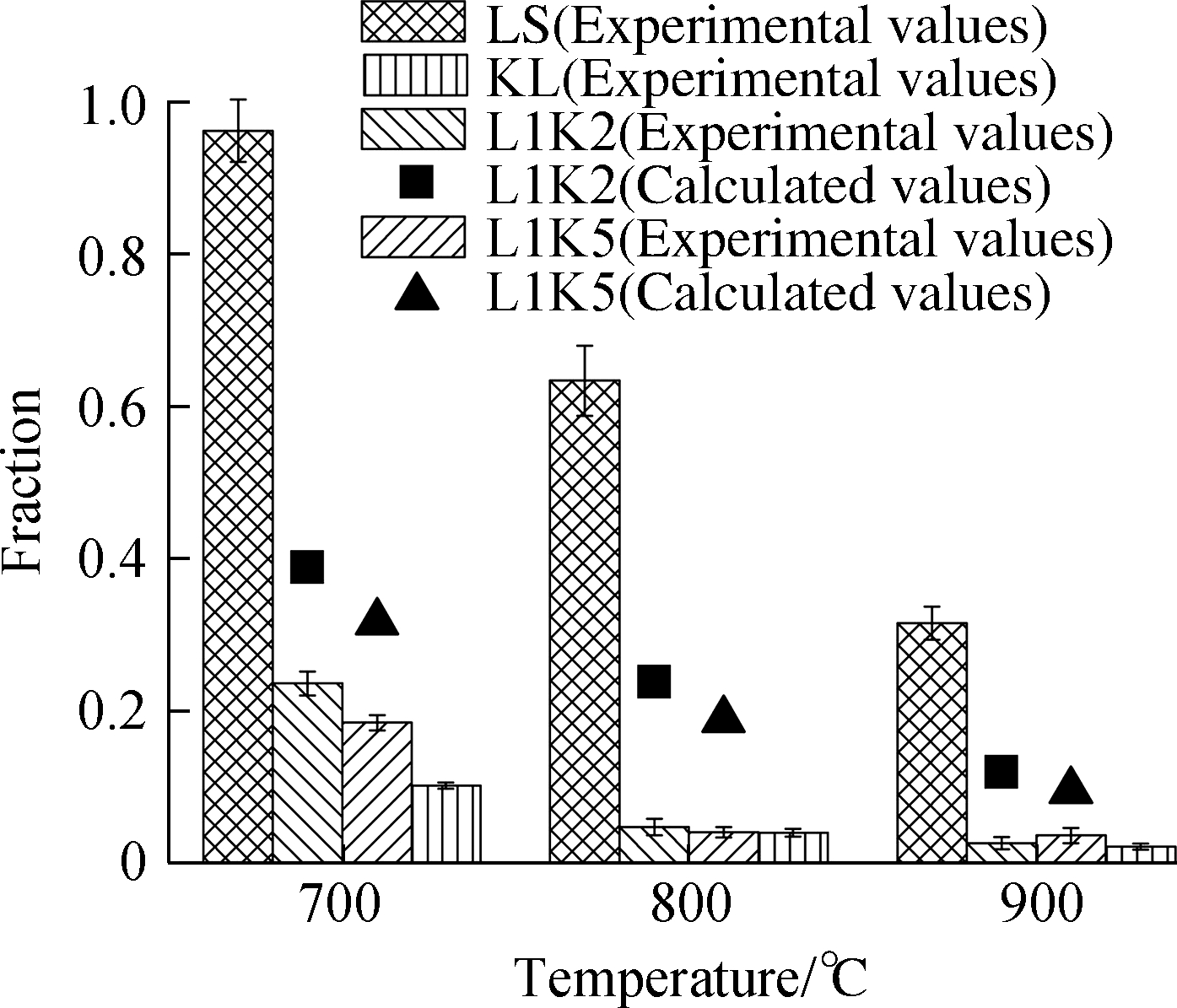
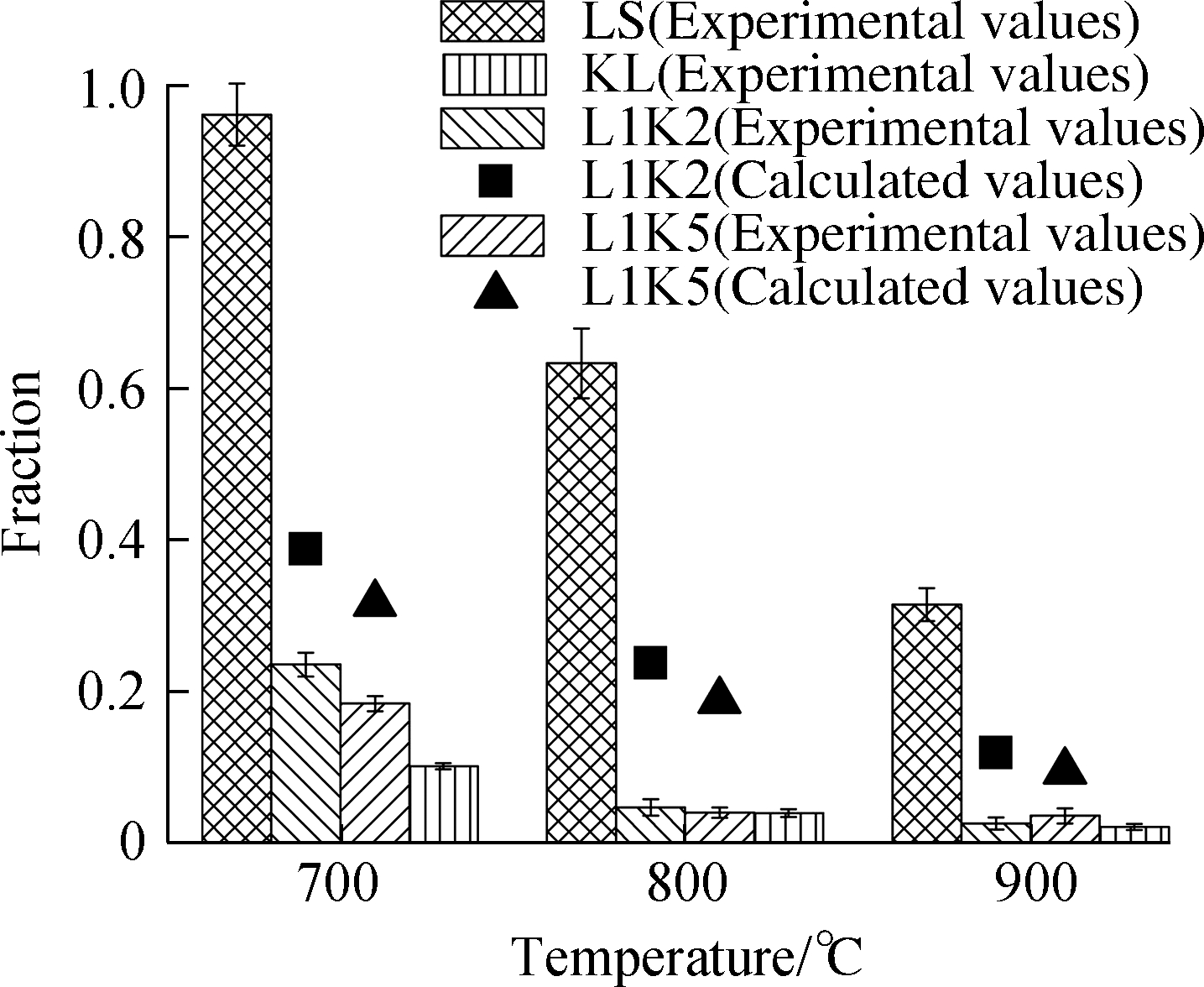
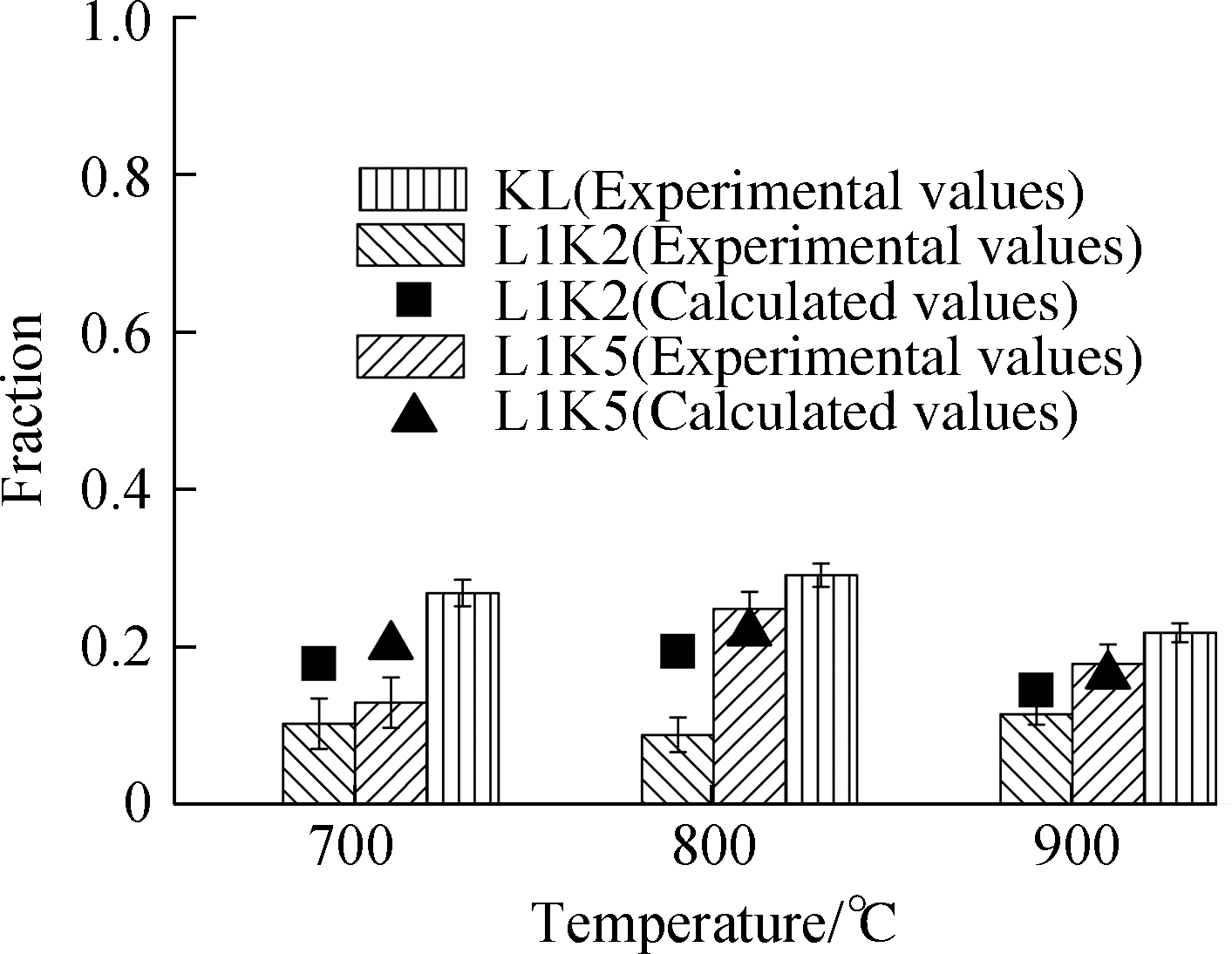
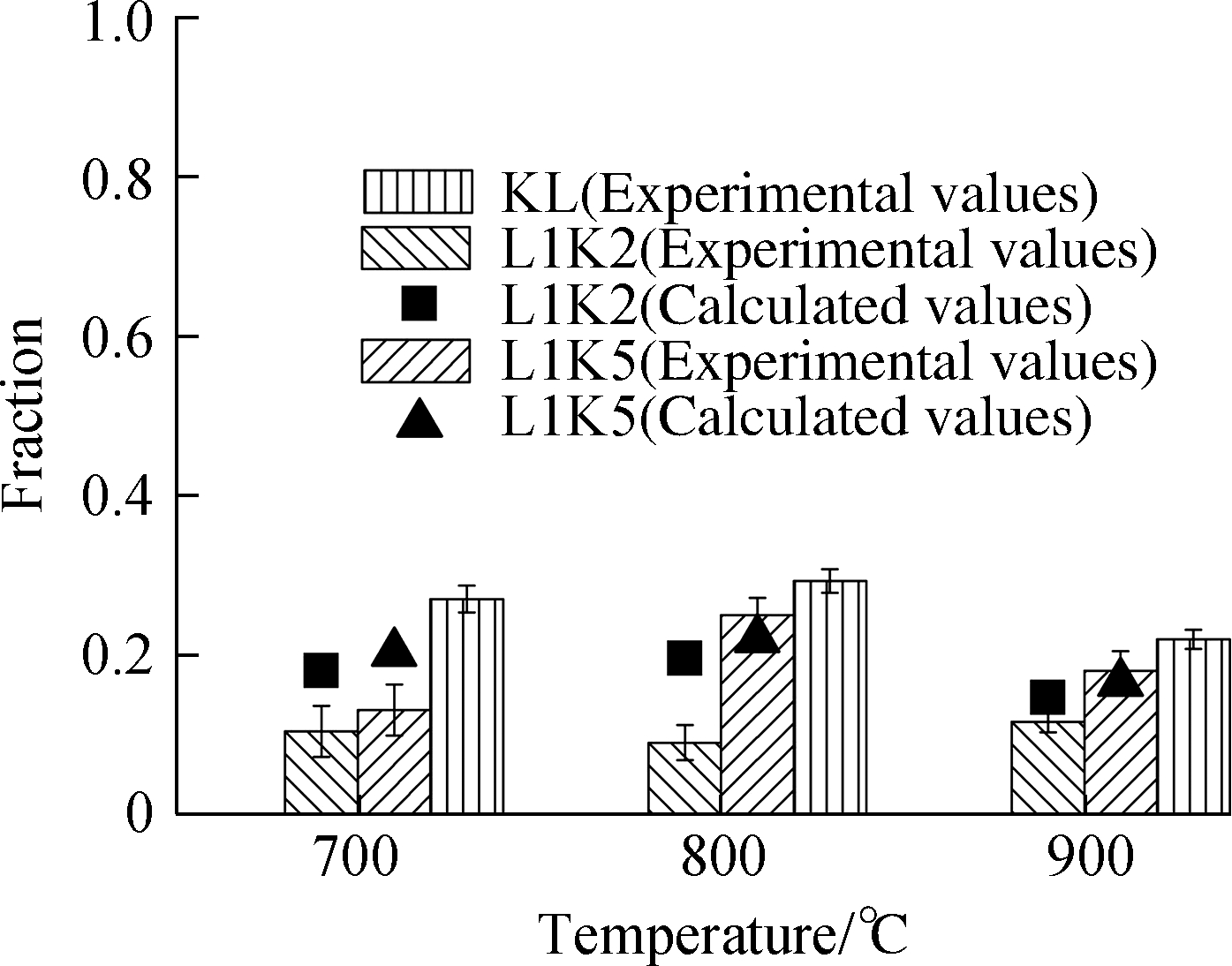
The distributions of heavy metals after the adsorption process are presented in Fig.3, where the experimental data are drawn as bars with error bars(standard deviation), and the calculated data of element distributions for the mixed sorbents are drawn as scatters.The calculated values were determined by the performances of kaolinite and limestone.Here, kaolinite and limestone in the same masses were performed separately; no interaction occurred between the two minerals in the mixed sorbents, so the effect of blending on the fixation behaviors can be evaluated.Their expression is defined as
(a)
(b)
(c)
(d)
(e)
(f)
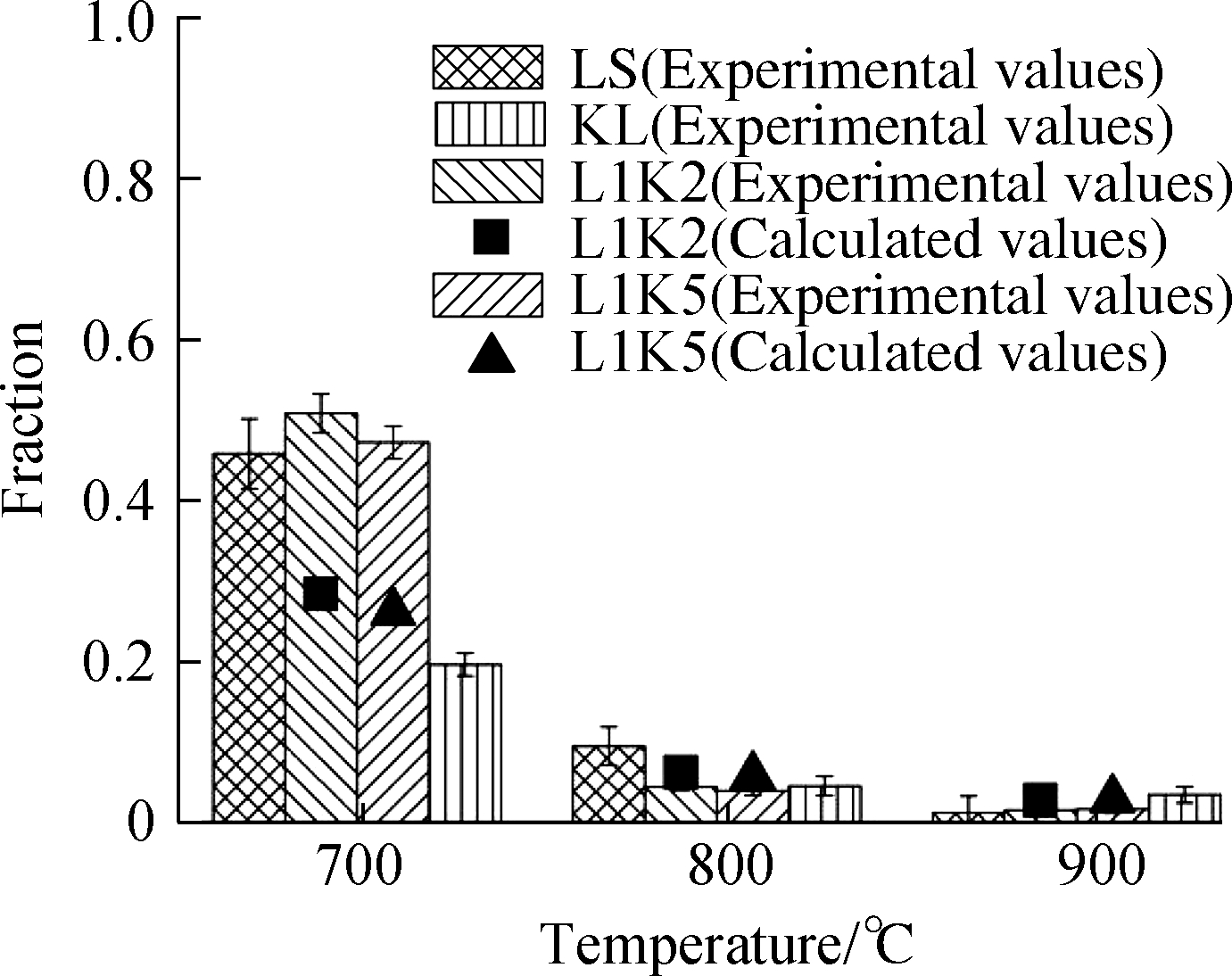
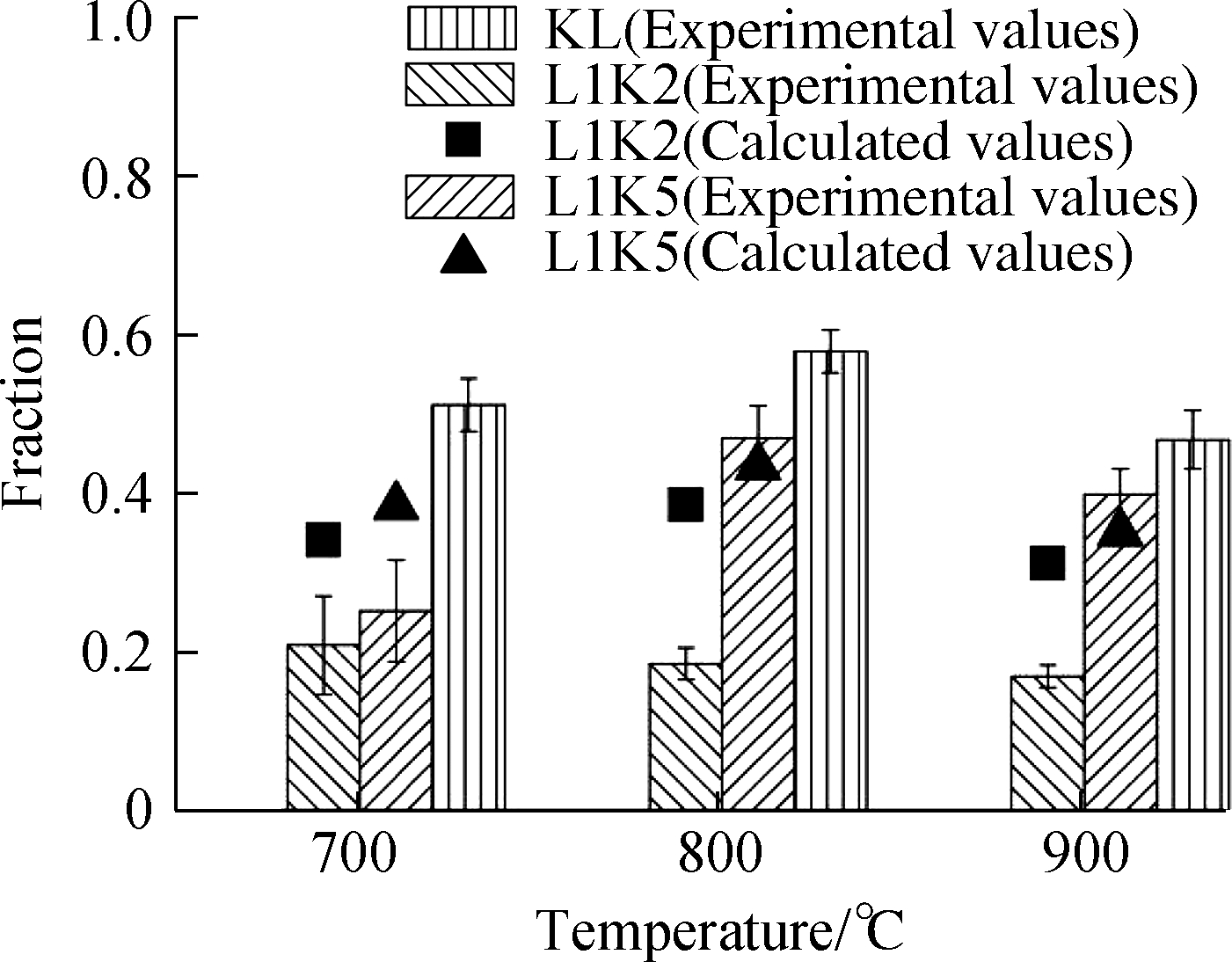
Fig.3 Adsorption efficiencies and form fractions for Cd and Pb.(a)Cd adsorption efficiency;(b)Cd leachable fraction;(c)Cd residual fraction;(d)Pb adsorption efficiency;(e)Pb leachable fraction;(f)Pb residual fraction
C=WKLVKL+WLSVLS
(4)
where C is the calculated value; WKL and WLS are the weight proportions of kaolinite and limestone, respectively; and VKL and VLS are the experimental values of kaolinite and limestone, respectively.
Limestone had a high cadmium adsorption efficiency of up to 0.95 at 700 ℃ but a much lower one of approximately 0.45 for lead.Then, the adsorption efficiencies both rapidly declined at higher temperatures caused by the negative movement of the equilibrium, and the capture ability for lead was almost even lost, but limestone still exceeded other sorbents in the cadmium capture[26].The capture rates by kaolinite were ranged between 0.24 and 0.35 for cadmium and 0.50 and 0.70 for lead, which also decreased with temperature increase due to the high saturated vapor pressure[34].
For the cadmium capture by the mixed sorbents, their efficiencies were lower than the two single sorbents and the calculated values.This finding indicates that the interaction between kaolinite and calcium hindered the cadmium sorption because of the calcium consumption via the reaction with kaolinite, and lime or limestone has a great ability to capture cadmium.Comparing the two mixed sorbents, L1K2 had a little higher efficiency than L1K5, while the total amounts of adsorbed cadmium by L1K2 were much lower than L1K5 at 800 and 900 ℃.
As for lead, the mixed sorbents differed from cadmium adsorption.At 700 ℃, L1K2 and L1K5 even performed slightly better than single kaolinite, and their experimental efficiencies were higher than the calculated ones, which means that the interaction between two kinds of minerals promoted lead sorption.However, when the temperatures reached 800 and 900 ℃, the efficiencies of L1K2 dramatically decreased and became much lower than the calculated values, indicating a heavy deactivation.Sorbent L1K5 showed close efficiency values to the calculated ones but was lower than those of KL.Therefore, calcium doping seems to slightly affect the total capture quantity of lead over 800 ℃.However, there were still potentially overlapped influences from the reaction between major minerals.
To explain this phenomenon, the adsorbed metals were divided into leached and residual forms.Of note, limestone or lime can be thoroughly leached by acids in the first step.Therefore, the fraction values of both metals in the leached form were equal to the total values, whereas those for the residual form were not detected at all for the sorbent LS.Generally, for sorbent with silicates, the adsorbed metal is first adsorbed onto the surface and then diffused inside the mineral lattice.This part of metal can almost be extracted by strong acid and is defined as the leached form here.Conversely, metals in the sintering material can be diffused and trapped in the cages of silicates, which can only be digested by hydrofluoric acid.Thus, the conversion from the leached form to the residual form was stimulated with the temperature increase for sorbents with silicates because of intensive melting.Consequently, as drawn in Fig.3, the reasons for the decreasing fractions of the leached form were the lower condensing capacity of metal vapors and the increasing conversion to the residual form induced by high temperatures.
Fractions of the cadmium leached form for both mixed sorbents were far less than the calculated values because LS contributed a significant part to the calculation.However, in the mixed sorbents, lime as an effective cadmium sorbent was reacted with silicates, according to the XRD analysis.As a result, only at 700 ℃, under the presence of calcite, the leached form of L1K2 was higher than that of L1K5, and the KL had the least amount of leached form.These values were then low at high temperatures due to the conversion to the residual form and the disappearance of lime or limestone.
The residual form was correlated with the proportions of kaolinite because of its silicon.The experimental values of L1K2 at all temperatures were lower than the calculated ones because an excessive amount of calcium could occupy the sites or vacancies inside the cage of silicates, which also inhibited the diffusion of heavy metals in the sorbent material and lessened the total adsorption efficiency.On the contrary, for L1K5 at 800 and 900 ℃, the experimental fraction values of the residual form were slightly beyond the calculated ones, indicating that the interaction between calcium and silicates helped the fixation of the metal.Its reason can be attributed to the Ca-low zones as was the only difference from the sorbent L1K2, where there was moderate melting induced by the doped calcium and metal diffusion could be enhanced.Moreover, there are still enough vacancies left for the fixation of heavy metals.
The form distributions of lead had very similar features with that of cadmium as shown in the right four pictures of Fig.3, except for the experimental condition of 700 ℃ for its leached form, whose fractions for the mixed sorbents were surprisingly over two single sorbents.It might be due to the reaction between kaolinite and calcium, which enhanced eutectic melting and helped the adsorption.However, the melting effect was not strong enough at that temperature to diffuse lead ions into the silicate cages for generating residual form, so the fractions of the residual form for both mixed sorbents were lower than the calculated ones[28,30].By contrast, lead may be more adherent to silicates than cadmium because its adsorption efficiency by kaolinite was much higher than that by cadmium, resulting in different performances between the two metals.
2.3 Discussion
Doping calcium into kaolinite caused multiple effects on the heavy metal chloride adsorption at a high temperature, where the uneven distribution of calcium generated different phases of silicates, i.e., Ca-rich zone and Ca-low zone.The mechanism scheme is drawn in Fig.4.
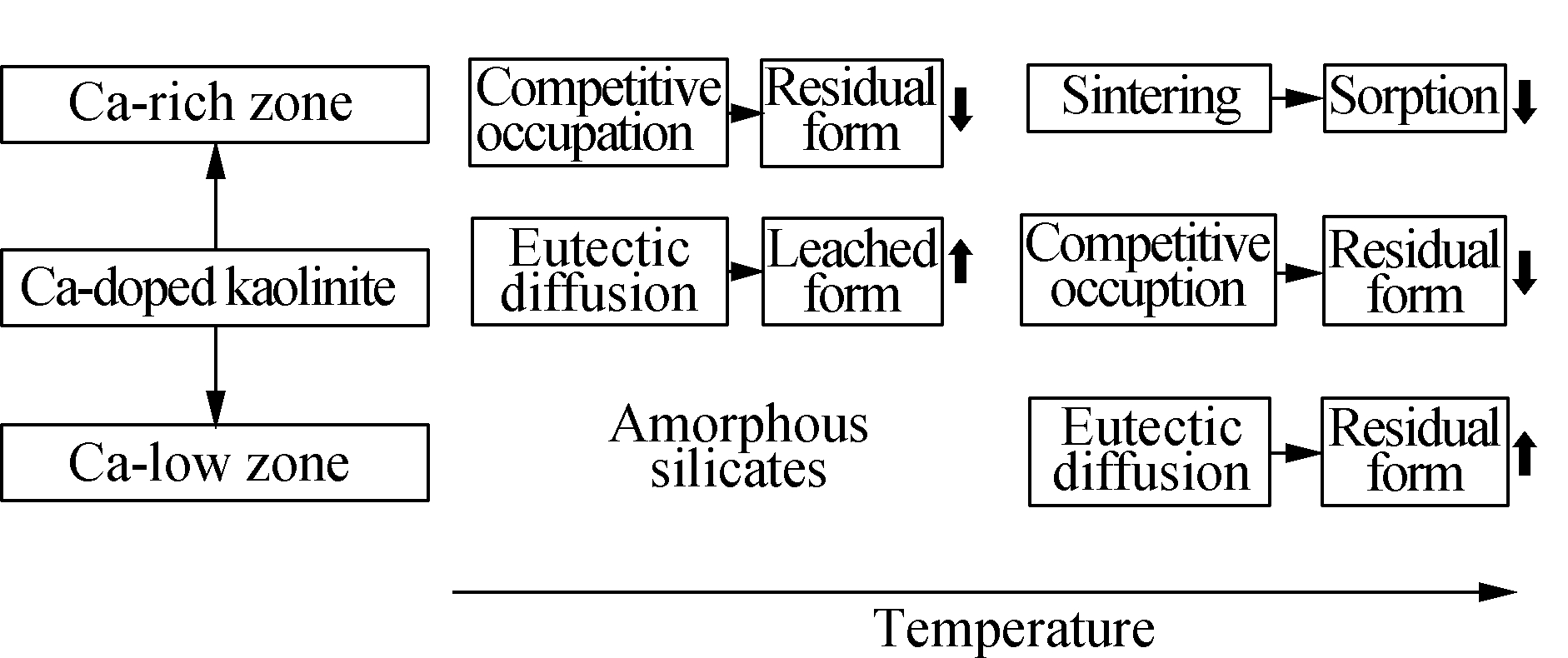
Fig.4 Mechanism of heavy metal capture by the calcium-kaolinite mixture
At a relatively low temperature, some calcium minerals were found in the Ca-rich zone and competitively occupied the inner position of silicates, which inhibited the conversion of the adsorbed heavy metals to their residual form.However, for lead, the reaction between calcium and silicates to form a low-melting-point matter, leading to a little eutectic melting and helping diffusion of lead into the sorbent, which consequently promoted the adsor-ption.If the amount of calcium doping was not sufficient, such as sorbent L1K5, then there was a meta-kaolinite-like matter left as the Ca-low zone because the temperature was not high enough for calcium diffusion to cover whole silicates.When the reaction temperature became higher, serious sintering in Ca-rich zones directly blocked the sorption ability, where the conversion of heavy metals to residual form also decreased.However, eutectic melting in the Ca-low zones enhanced the transformations of both heavy metals into the residual form.
For a potential application of metal sorption in a furnace, blending as mall amount of calcium into kaolinite can help heavy metal chlorides capture and gain the adsorption product with low toxicity.A well-dispersed mixture would be preferable to avoid the formation of Ca-rich zones as far as possible.On the contrary, any unsuitable method and proportion of blending calcium-based minerals and silicates as combustion additives may eliminate the sorption properties of both single sorbents.
3 Conclusions
1)This experimental study investigated gaseous PbCl2 and CdCl2 adsorptions by calcium-doped kaolinite at high temperatures.Limestone was blended with kaolinite, and new calcium minerals were generated at high temperatures.Two types of phases in the sorbent, named as Ca-rich zone and Ca-low zone, were clarified by SEM-EDS.
2)Compared with two single sorbents of kaolinite and limestone, the capture efficiencies by the mixed sorbents were lower, only except for lead at 700 ℃.The weaker adsorption resulted from the consumption of lime or limestone to react with kaolinite for cadmium and the lower proportions of silicates for the lead.
3)Interactions between calcium and aluminum silicates had diverse impacts on metal sorption.The formation and eutectic effect of low-melting-point substances with calcium could enhance the diffusion of heavy metals and their conversions to a more stable form, but they could also block the pore structure by serious sintering at higher temperatures and occupy the reactive sites to inhibit the adsorption.Therefore, the attempt of mixing kaolinite with calcium for heavy metal adsorption can cause either positive or negative results, which depends on the blending condition and reaction temperature.
[1] Zhou C, Liu G, Yan T, et al.Transformation behavior of mineral composition and trace elements during coal gangue combustion[J].Fuel, 2012, 97:644-650.DOI:10.1016/j.fuel.2012.02.027.
[2] Shadman F, Uberoi M.Simultaneous condensation and reaction of metal compound vapors in porous solids[J].Industrial and Engineering Chemistry Research, 1991, 30(4):624-631.DOI:10.1021/ie00052a004.
[3] Davis S B, Gale T K, Wendt J, et al.Multicomponent coagulation and condensation of toxic metals in combustors[J].Symposium(International)on Combustion, 1998, 27(2):1785-1791.DOI:10.1016/S0082-0784(98)80020-9.
[4] Xiao H, Chen Y, Li L, et al.Study on the volatilization behavior of heavy metals(As, Cd)during co-processing in furnaces and boilers[J].Environmental Engineering Science, 2017, 34(5):333-342.DOI:10.1089/ees.2016.0144.
[5] Liu C, Huang Y, Wang X, et al.Dynamic experimental investigation on the volatilization behavior of lead and cadmium in the simulated municipal solid waste(MSW)influenced by sulfur compounds during incineration[J].Energy and Fuels, 2017, 31(1):847-853.DOI:10.1021/acs.energyfuels.6b01315.
[6] Tissari J, Sippula O, Torvela T, et al.Zinc nanoparticle formation and physicochemical properties in wood combustion—Experiments with zinc-doped pellets in a small-scale boiler[J].Fuel, 2015, 143:404-413.DOI:10.1016/j.fuel.2014.11.076.
[7] Yu J, Sun L, Wang B, et al.Study on the behavior of heavy metals during thermal treatment of municipal solid waste(MSW)components[J].Environmental Science and Pollution Research, 2016, 23(1):253-265.DOI:10.1007/s11356-015-5644-7.
[8] Lu P, Huang Q, Bourtsalas A C, et al.Review on fate of chlorine during thermal processing of solid wastes[J].Journal of Environmental Sciences(China), 2019, 78:13-28.DOI:10.1016/j.jes.2018.09.003.
[9] Linak W P, Wendt J.Toxic metal emissions from incineration:Mechanisms and control[J].Progress in Energy and Combustion Science, 1993, 19(2):145-185.DOI:10.1016/0360-1285(93)90014-6.
[10] Yao H, Naruse I.Using sorbents to control heavy metals and particulate matter emission during solid fuel combustion[J].Particuology, 2009, 7(6):477-482.DOI:10.1016/j.partic.2009.06.004.
[11] Scotto M V, Uberoi M, Peterson, T W, et al.Metal capture by sorbents in combustion processes[J].Fuel Processing Technology, 1994, 39(1/2/3):357-372.DOI:10.1016/0378-3820(94)90192-9.
[12] Wang J, Takarada T.Fixation of lead chloride on kaolinite and bentonite at temperatures between 550 and 950 ℃[J].Industrial and Engineering Chemistry Research, 2000, 39(2):335-341.DOI:10.1021/ie9905097.
[13] Xu Y, Liu X, Wang H, et al.Influences of in-furnace kaolin addition on the formation and emission characteristics of PM2.5 in a 1000 MW coal-fired power station[J].Environmental Science and Technology, 2018, 52(15):8718-8724.DOI:10.1021/acs.est.8b02251.
[14] Zhang X, Liu H, Xing H, et al.Improved sodium adsorption by modified kaolinite at high temperature using intercalation-exfoliation method[J].Fuel, 2017, 191:198-203.DOI:10.1016/j.fuel.2016.11.067.
[15] Wang G, Jensen P A, Wu H, et al.Potassium capture by kaolin, Part 1:KOH[J].Energy and Fuels, 2018, 32(2):1851-1862.DOI:10.1021/acs.energyfuels.7b03645.
[16] Wang X, Huang Y, Zhong Z, et al.Control of inhalable particulate lead emission from incinerator using kaolin in two addition modes[J].Fuel Processing Technology, 2014, 119:228-235.DOI:10.1016/j.fuproc.2013.11.012.
[17] Wendt J, Lee S.High-temperature sorbents for Hg, Cd, Pb, and other trace metals:Mechanisms and applications[J].Fuel, 2010, 89(4):894-903.DOI:10.1016/j.fuel.2009.01.028.
[18] Yu S, Zhang C, Ma L, et al.Experimental and DFT studies on the characteristics of PbO/PbCl2 adsorption by Si/Al-based sorbents in the simulated flue gas[J].Journal of Hazardous Materials, 2021, 407:123617.DOI:10.1016/j.jhazmat.2020.124742.
[19] Wang X, Huang Y, Zhong Z, et al.Theoretical investigation of cadmium vapor adsorption on kaolinite surfaces with DFT calculations[J].Fuel, 2016, 166:333-339.DOI:10.1016/j.fuel.2015.11.004.
[20] Wang X, Huang Y, Pan Z, et al.Theoretical investigation of lead vapor adsorption on kaolinite surfaces with DFT calculations[J].Journal of Hazardous Materials, 2015, 295:43-54.DOI:10.1016/j.jhazmat.2015.03.020.
[21] Wang X, Chen M, Liu C, et al.Typical gaseous semi-volatile metals adsorption by meta-kaolinite:A DFT study[J].International Journal of Environmental Research and Public Health, 2018, 15(10):1-14.DOI:10.3390/ijerph15102154.
[22] Ke C, Ma X, Tang Y, et al.Effects of natural and modified calcium-based sorbents on heavy metals of food waste under oxy-fuel combustion[J].Bioresource Technology, 2019, 271:251-257.DOI:10.1016/j.biortech.2018.09.109.
[23] Zheng W, Ma X, Tang Y, et al.Heavy metal control by natural and modified limestone during wood sawdust combustion in a CO2/O2 atmosphere[J].Energy and Fuels, 2018, 32(2):2630-2637.DOI:10.1021/acs.energyfuels.7b03365.
[24] Wang S, He P, Shao L, et al.Multifunctional effect of Al2O3, SiO2 and CaO on the volatilization of PbO and PbCl2 during waste thermal treatment[J].Chemosphere, 2016, 161:242-250.DOI:10.1016/j.chemosphere.2016.07.020.
[25] Wang X, Huang Y, Liu C, et al.Dynamic volatilization behavior of Pb and Cd during fixed bed waste incineration:Effect of chlorine and calcium oxide[J].Fuel, 2017, 192:1-9.DOI:10.1016/j.fuel.2016.12.002.
[26] Zha J, Zhu Z, Huang Y, et al.Gaseous CdCl2 and PbCl2 adsorption by limestone at high temperature:Mechanistic study through experiments and theoretical calculation[J].Applied Surface Science, 2021, 555:149669.DOI:10.1016/j.apsusc.2021.149669.
[27] Chen D, Hu H, Xu Z, et al.Findings of proper temperatures for arsenic capture by CaO in the simulated flue gas with and without SO2[J].Chemical Engineering Journal, 2015, 267:201-206.DOI:10.1016/j.cej.2015.01.035.
[28] Liu X, Xu Y, Qi J, et al.Effects of kaolin-limestone blended additive on the formation and emission of particulate matter:Field study on a 1 000 MW coal-firing power station[J].Journal of Hazardous Materials, 2020,399:DOI:10.1016/j.jhazmat.2020.123091.
[29] Zha J, Huang Y, Clough P, et al.Desulfurization using limestone during sludge incineration in a fluidized bed furnace:Increased risk of particulate matter and heavy metal emissions[J].Fuel, 2020, 273:117614.DOI:10.1016/j.fuel.2020.117614.
[30] Zha J, Huang Y, Xia W, et al.Effect of mineral reaction between calcium and aluminosilicate on heavy metal behavior during sludge incineration[J].Fuel, 2018, 229:241-247.DOI:10.1016/j.fuel.2018.05.015.
[31] Folgueras M B, Díaz R M, Xiberta J, et al.Effect of inorganic matter on trace element behavior during combustion of coal-sewage sludge blends[J].Energy and Fuels, 2007, 21(2):744-755.DOI:10.1021/ef060536r.
[32] Wang T, Xue Y, Zhou M, et al.Effect of addition of rice husk on the fate and speciation of heavy metals in the bottom ash during dyeing sludge incineration[J].Journal of Cleaner Production, 2020, 244:DOI:10.1016/j.jclepro.2019.118851.
[33] Bozaghian M, Rebbling A, Larsson S, et al.Combustion characteristics of straw stored with CaCO3 in bubbling fluidized bed using quartz and olivine as bed materials[J].Applied Energy, 2018, 212:1400-1408.DOI:10.1016/j.apenergy.2017.12.112.
[34] Zha J, Huang Y, Clough P, et al.Green production of a novel sorbent from kaolin for capturing gaseous PbCl2 in a furnace[J].Journal of Hazardous Materials, 2021, 404:124045.DOI:10.1016/j.jhazmat.2020.124045.
[35] Okada K, Watanabe N, Jha K V, et al.Effects of grinding and firing conditions on CaAl2Si2O8 phase formation by solid-state reaction of kaolinite with CaCO3[J].Applied Clay Science, 2003, 23(5/6):329-336.DOI:10.1016/S0169-1317(03)00132-7.