Nitrate ![]() is often the focus in the nitrogen(N)pollution investigation of surface water and shallow groundwater[1].Due to its stability,
is often the focus in the nitrogen(N)pollution investigation of surface water and shallow groundwater[1].Due to its stability, ![]() degrades slowly under natural conditions, leading to massive and persistent accumulation[2].Excess
degrades slowly under natural conditions, leading to massive and persistent accumulation[2].Excess ![]() stimulates algal growth, resulting in eutrophication and hypoxia[3-4].High
stimulates algal growth, resulting in eutrophication and hypoxia[3-4].High ![]() concentrations in drinking water have also been linked to diabetes, spontaneous abortion, thyroid problems, and stomach cancer[5].To ensure human health, the World Health Organization established the maximum contaminant level(MCL)of 50 mg/L for
concentrations in drinking water have also been linked to diabetes, spontaneous abortion, thyroid problems, and stomach cancer[5].To ensure human health, the World Health Organization established the maximum contaminant level(MCL)of 50 mg/L for ![]() in drinking water; however, many countries have still exceeded this MCL for
in drinking water; however, many countries have still exceeded this MCL for ![]() from drinking water sources in recent years[6-8].To remove
from drinking water sources in recent years[6-8].To remove ![]() from water, chemical reduction methods with a fast reaction rate and easy operation are widely adopted.Conventional chemical denitrification includes metal reduction and catalytic reduction.Chemical reduction methods use iron[9], aluminum[10], zinc, and other zerovalent metal as electron donors, and conventional chemical denitrification combines catalysts(usually metal-doped or carbon-doped semiconductor materials[11])and hole scavengers(usually hydrogen or formic acid[HCOOH])to obtain electronic interaction and electron transfer.Current research on chemical denitrification mainly aims to control the direction and degree of
from water, chemical reduction methods with a fast reaction rate and easy operation are widely adopted.Conventional chemical denitrification includes metal reduction and catalytic reduction.Chemical reduction methods use iron[9], aluminum[10], zinc, and other zerovalent metal as electron donors, and conventional chemical denitrification combines catalysts(usually metal-doped or carbon-doped semiconductor materials[11])and hole scavengers(usually hydrogen or formic acid[HCOOH])to obtain electronic interaction and electron transfer.Current research on chemical denitrification mainly aims to control the direction and degree of ![]() reduction to avoid nitrite
reduction to avoid nitrite ![]() or ammonia(NH3)formation and improve gaseous N selectivity.
or ammonia(NH3)formation and improve gaseous N selectivity.
Recently, advanced reduction processes(ARPs)that produce reducing radicals to destroy contaminants by activating reagents have been widely adopted in the field of water treatment.Compared with traditional chemical methods, ARPs have the advantages of higher removal efficiency, more stable performance over a wide pH range, and easier combination with ultraviolet(UV)disinfection[12-13].![]() has been proven to be removable by
has been proven to be removable by ![]() ARP according to Bensalah et al.[14], but sulfur and ammonium were introduced to the system.A suitable ARP for
ARP according to Bensalah et al.[14], but sulfur and ammonium were introduced to the system.A suitable ARP for ![]() removal from drinking water should ensure that the products of reducing radicals are ultimately removed as well, and the carbon dioxide anion radical(·
removal from drinking water should ensure that the products of reducing radicals are ultimately removed as well, and the carbon dioxide anion radical(·![]() generated from organic acids or salts is an appropriate choice.·
generated from organic acids or salts is an appropriate choice.·![]() is a strongly reducing radical with a REDOX potential of E(CO2/·
is a strongly reducing radical with a REDOX potential of E(CO2/·![]() V[15].After being oxidized,·
V[15].After being oxidized,·![]() is converted into CO2 that easily discharges into the atmosphere.HCOOH is regarded as the most favorable·
is converted into CO2 that easily discharges into the atmosphere.HCOOH is regarded as the most favorable·![]() provider because of its simple carboxylic acid structure[16].Gu et al.[17] developed
provider because of its simple carboxylic acid structure[16].Gu et al.[17] developed ![]() ARP for carbon tetrachloride degradation, through which·
ARP for carbon tetrachloride degradation, through which·![]() was rapidly produced.Chen et al.[18] adopted UV/H2O2/HCOOH for
was rapidly produced.Chen et al.[18] adopted UV/H2O2/HCOOH for ![]() reduction and achieved approximately 100%
reduction and achieved approximately 100% ![]() removal, as well as a maximum gaseous N product selectivity of 80%.
removal, as well as a maximum gaseous N product selectivity of 80%.
Inspired by the aforementioned systems, this study aims to develop a highly efficient denitrification process utilizing ARPs without producing other pollutants.Herein, a simple system with UV as the activation method and HCOOH alone as the reducing agent was established.
HCOOH, sodium nitrate, and phosphate were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd.(Tianjin, China).5,5-Dimethyl-1-pyrrolidine N-oxide(DMPO)was purchased from Aladdin(Shanghai, China).The other chemicals, such as potassium chloride, potassium bicarbonate, and potassium dihydrogen phosphate, were provided by Sinopharm Group Chemical Reagent Co., Ltd.(Shanghai, China).All chemicals were of analytical grade.
Experiments were conducted in a 250 mL quartz photochemical reactor with an inner condensing trap.All solutions were prepared with deionized deoxygenated water with a resistivity of more than 18 MΩ at room temperature.The initial concentration of ![]() was 50 mg/L(3.57 mmol/L).Solutions were first aerated with N2 or O2 for five min and then subjected to the REDOX reaction under UV light.UV irradiation was conducted using high-pressure mercury lamps emitting polychromatic UV light between 200 and 650 nm.The lamps were warmed up for 15 min to reach a constant output before starting the irradiation experiments.Samples were taken every 15 min, and the reaction duration was 90 min.
was 50 mg/L(3.57 mmol/L).Solutions were first aerated with N2 or O2 for five min and then subjected to the REDOX reaction under UV light.UV irradiation was conducted using high-pressure mercury lamps emitting polychromatic UV light between 200 and 650 nm.The lamps were warmed up for 15 min to reach a constant output before starting the irradiation experiments.Samples were taken every 15 min, and the reaction duration was 90 min.
The analysis of N-containing compounds was performed using a UV-visible spectrophotometer(UV-1800PC)according to the national standards HJ/T 346—2007 ![]() HJ 535—2009(NH3), and HJ 636—2012.Total organic carbon(TOC)was measured using a TOC analyzer(OLTOC1030W).The concentration of HCOOH was measured by sodium hydroxide titration.Radicals were detected by electron paramagnetic resonance(EPR)with a Bruker A300 EPR spectrometer.Each sample was mixed with the spin trapping agent DMPO and injected into capillary tubes with puncture needles for detection.
HJ 535—2009(NH3), and HJ 636—2012.Total organic carbon(TOC)was measured using a TOC analyzer(OLTOC1030W).The concentration of HCOOH was measured by sodium hydroxide titration.Radicals were detected by electron paramagnetic resonance(EPR)with a Bruker A300 EPR spectrometer.Each sample was mixed with the spin trapping agent DMPO and injected into capillary tubes with puncture needles for detection.
The denitrification efficiencies of UV/HCOOH/N2, UV/HCOOH/O2, and UV/HCOOH were compared.The initial concentration of HCOOH was 10 mmol/L, and the power input of UV light was 125 W.The initial dissolved oxygen(DO)concentration of the solution was less than 0.1 mg/L under N2 aeration, 26.0 mg/L under O2 aeration, and 7.8 mg/L without aeration.The removal ratio of ![]() at 90 min was 100%, 93.7%, and 98.9%.Reducing radicals can be rapidly consumed by O2, leading to a decrease in the
at 90 min was 100%, 93.7%, and 98.9%.Reducing radicals can be rapidly consumed by O2, leading to a decrease in the ![]() removal ratio as the DO concentration increases.This finding indicates that the reducing atmosphere promotes
removal ratio as the DO concentration increases.This finding indicates that the reducing atmosphere promotes ![]() reduction.Therefore, the basic condition in this study was determined to be UV/HCOOH/N2.
reduction.Therefore, the basic condition in this study was determined to be UV/HCOOH/N2.
Then, the effect of the initial HCOOH concentration(C0)on denitrification was investigated for optimal dosage determination, and the results are presented in Fig.1.As the C0(HCOOH)concentration increased from 1 mol/L to 9 mmol/L, the reduction ratio of ![]() increased from 22.4% to 98.7% within 60 min, and the gas conversion ratio increased from 1.0% to 97.6%.At the end of the reaction, the reduction and gas conversion ratios of
increased from 22.4% to 98.7% within 60 min, and the gas conversion ratio increased from 1.0% to 97.6%.At the end of the reaction, the reduction and gas conversion ratios of ![]() were 99.9% and 99.8%, respectively, with C0(HCOOH)=9 mM, which was the highest.The reduction and gas conversion ratios were numerically similar, indicating that the dominant product of reduced
were 99.9% and 99.8%, respectively, with C0(HCOOH)=9 mM, which was the highest.The reduction and gas conversion ratios were numerically similar, indicating that the dominant product of reduced ![]() was gas.
was gas.
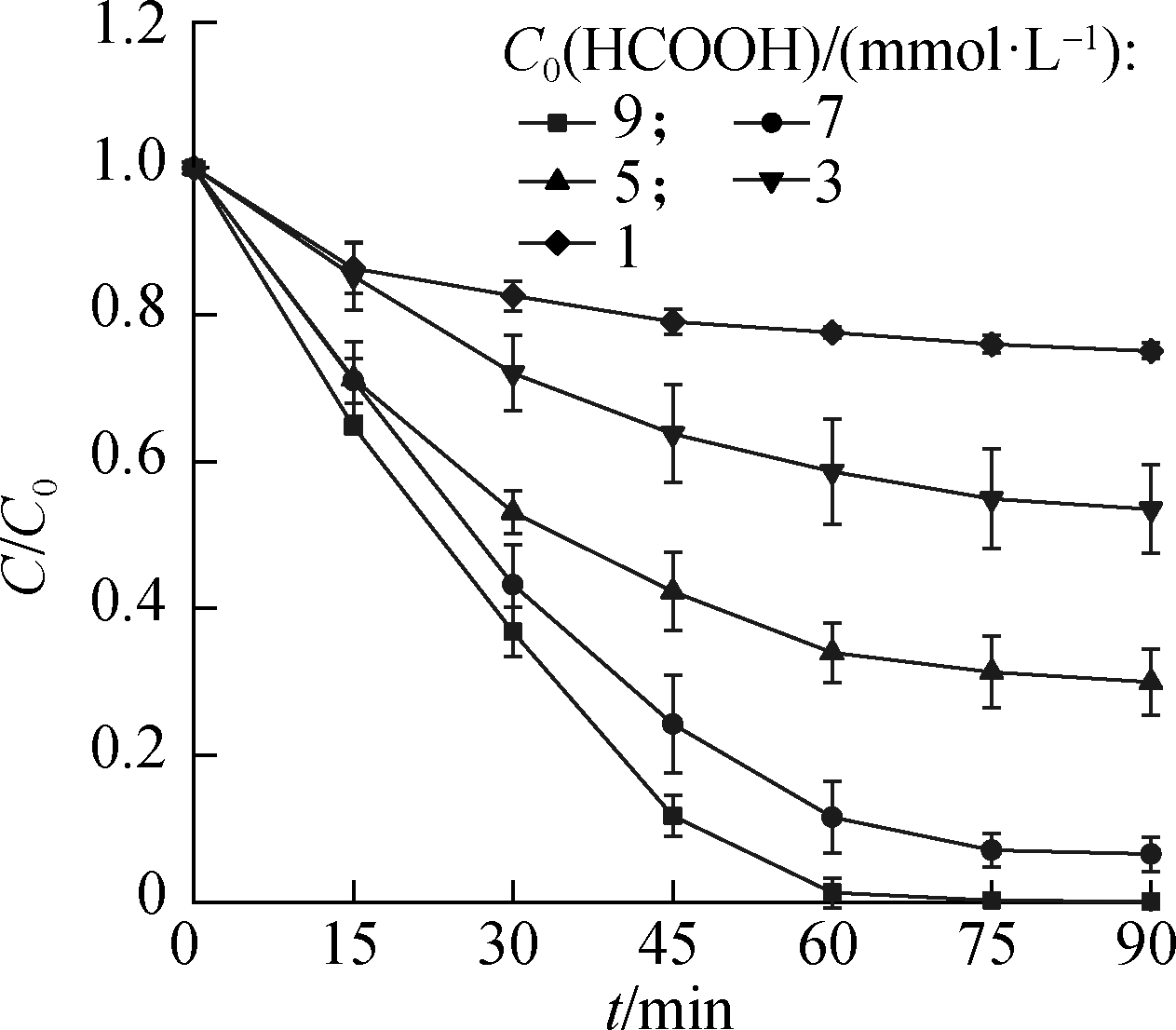
(a)
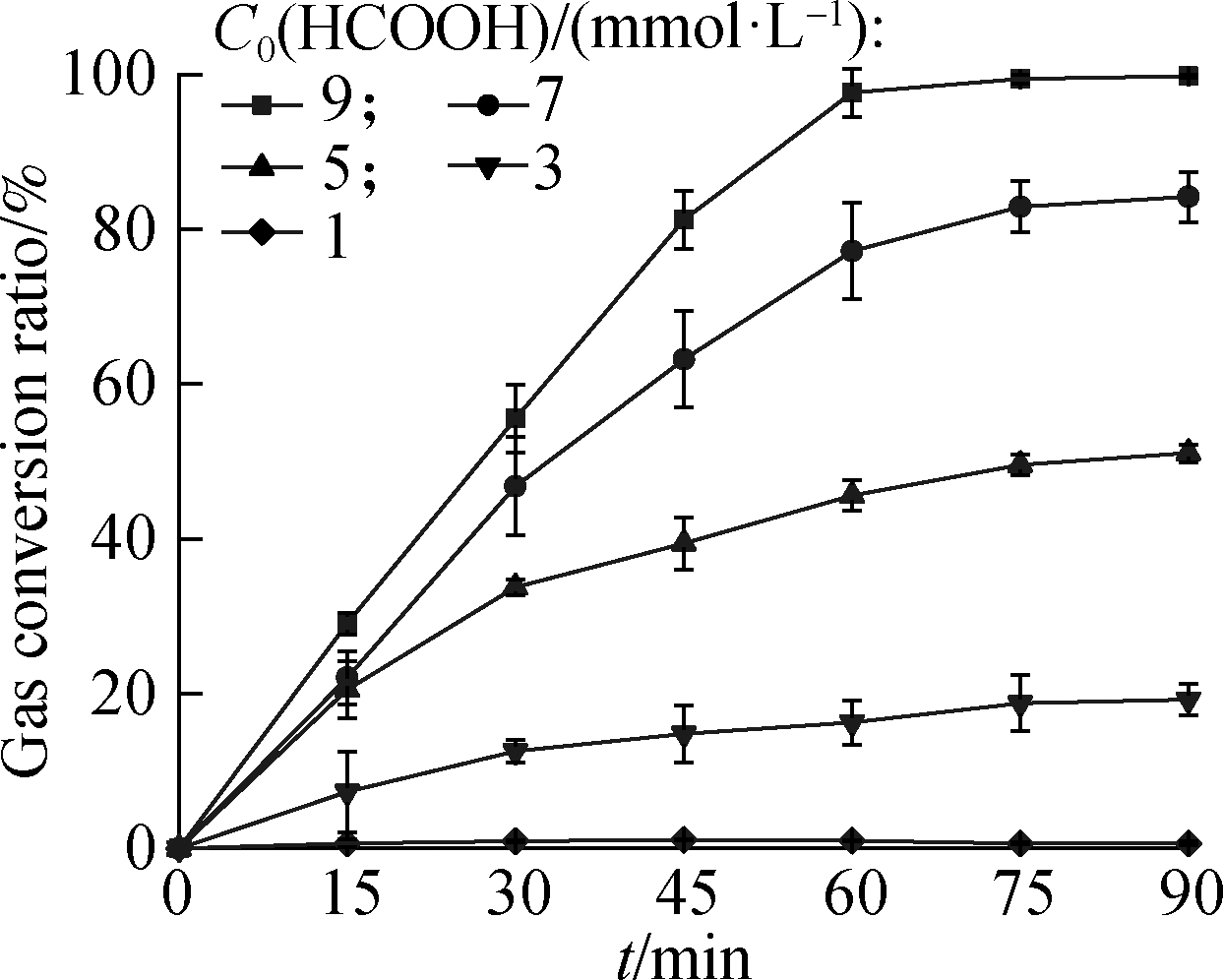
(b)
Fig.1 Effect of C0(HCOOH)concentration on ![]() reduction.(a)Reduction ratio;(b)Gas conversion ratio
reduction.(a)Reduction ratio;(b)Gas conversion ratio
The TOC concentration and the residual HCOOH concentration were detected for toxicity evaluation.Fig.2(a)presents the dramatic decrease in TOC concentration with time under different C0(HCOOH)concentrations, indicating that HCOOH was depleted continuously with gas as the dominant product.When the C0(HCOOH)concentration was less than 7 mmol/L, the eventual TOC concentration was lower than the limit set in the Hygienic Standard for Drinking Water(GB 5749—2006; i.e., 5 mg/L), and the highest ![]() removal and gas conversion ratios reached 93.5% and 84.2%, respectively.When the C0(HCOOH)concentration was 9 mmol/L, the removal and gas conversion ratios exceeded 99.8%, but the final TOC concentration was the highest(i.e., 11.9 mg/L).In short,
removal and gas conversion ratios reached 93.5% and 84.2%, respectively.When the C0(HCOOH)concentration was 9 mmol/L, the removal and gas conversion ratios exceeded 99.8%, but the final TOC concentration was the highest(i.e., 11.9 mg/L).In short, ![]() reduction by UV/HCOOH/N2 ARP involves the risk of secondary pollution.If the requirement for
reduction by UV/HCOOH/N2 ARP involves the risk of secondary pollution.If the requirement for ![]() removal is strict, then excessive HCOOH may cause the TOC concentration in the solution at the end of the reaction to exceed the limit of the sanitary standards for domestic drinking water.
removal is strict, then excessive HCOOH may cause the TOC concentration in the solution at the end of the reaction to exceed the limit of the sanitary standards for domestic drinking water.
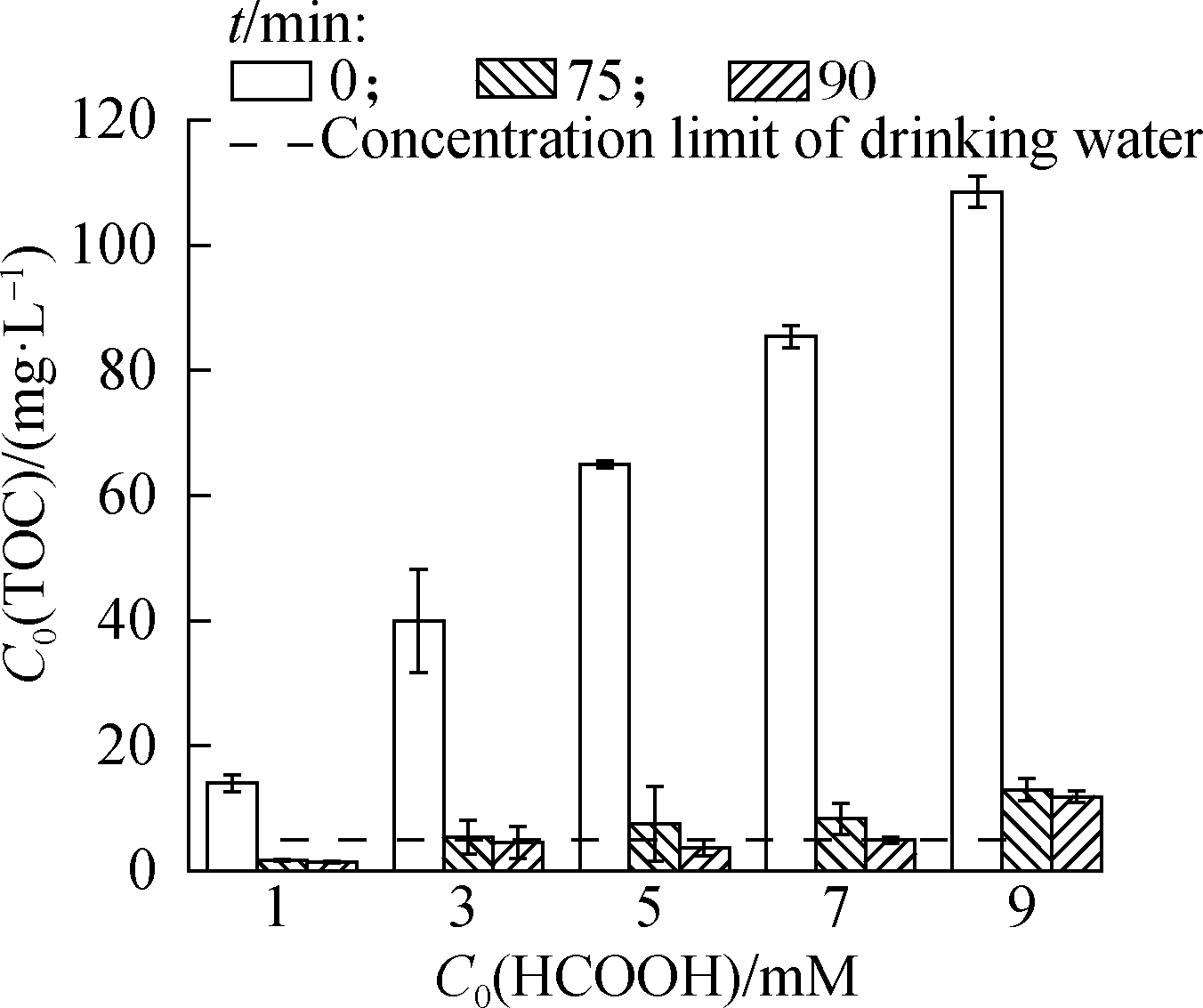
(a)
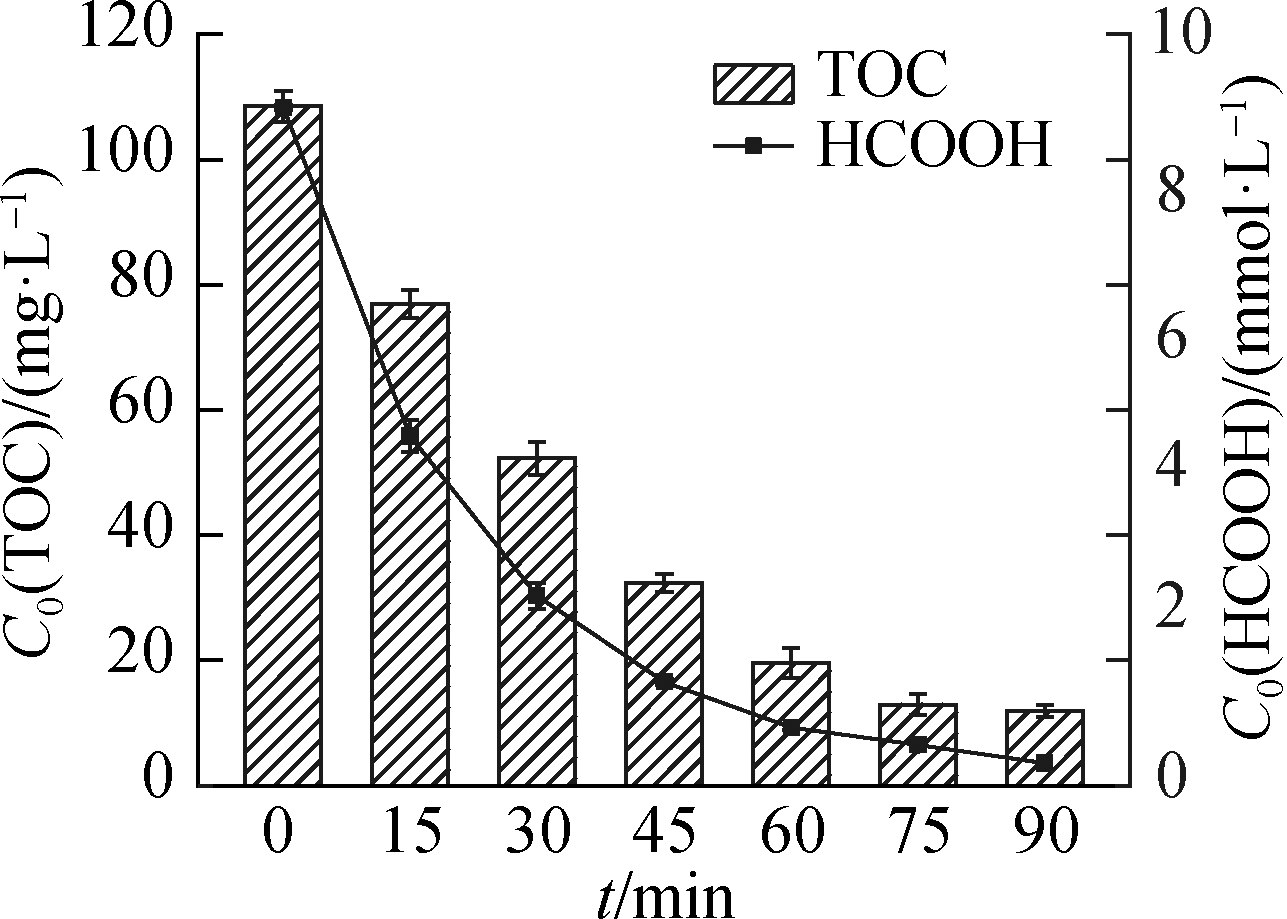
(b)
Fig.2 TOC and HCOOH evaluation.(a)TOC concentration under different C0(HCOOH)concentrations;(b)TOC and residual HCOOH concentrations when C0(HCOOH)=9 mM
To guarantee the reduction effect,the C0(HCOOH)concentration used in subsequent studies was 9 mmol/L, and the trends of the TOC and HCOOH concentrations were further analyzed(see Fig.2(b)).At the end of UV illumination, more than 96.7% of HCOOH was consumed, and the residual HCOOH concentration was at a negligible level(0.3 mmol/L).
In addition to gas, the possible by-products of ![]() in the UV/HCOOH/N2 reduction process include
in the UV/HCOOH/N2 reduction process include ![]() and NH3.Thus, their formation was quantitatively analyzed.
and NH3.Thus, their formation was quantitatively analyzed.
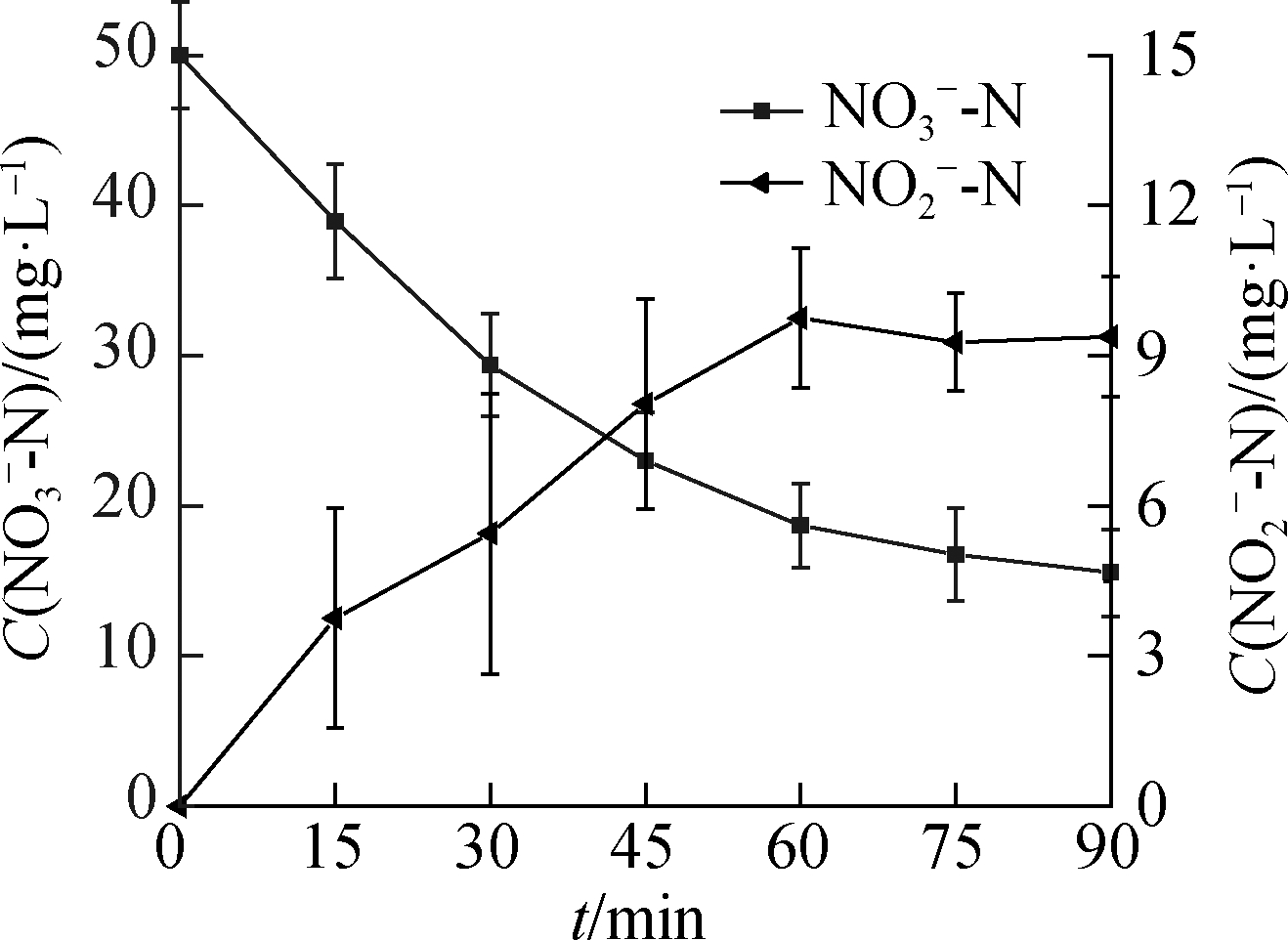
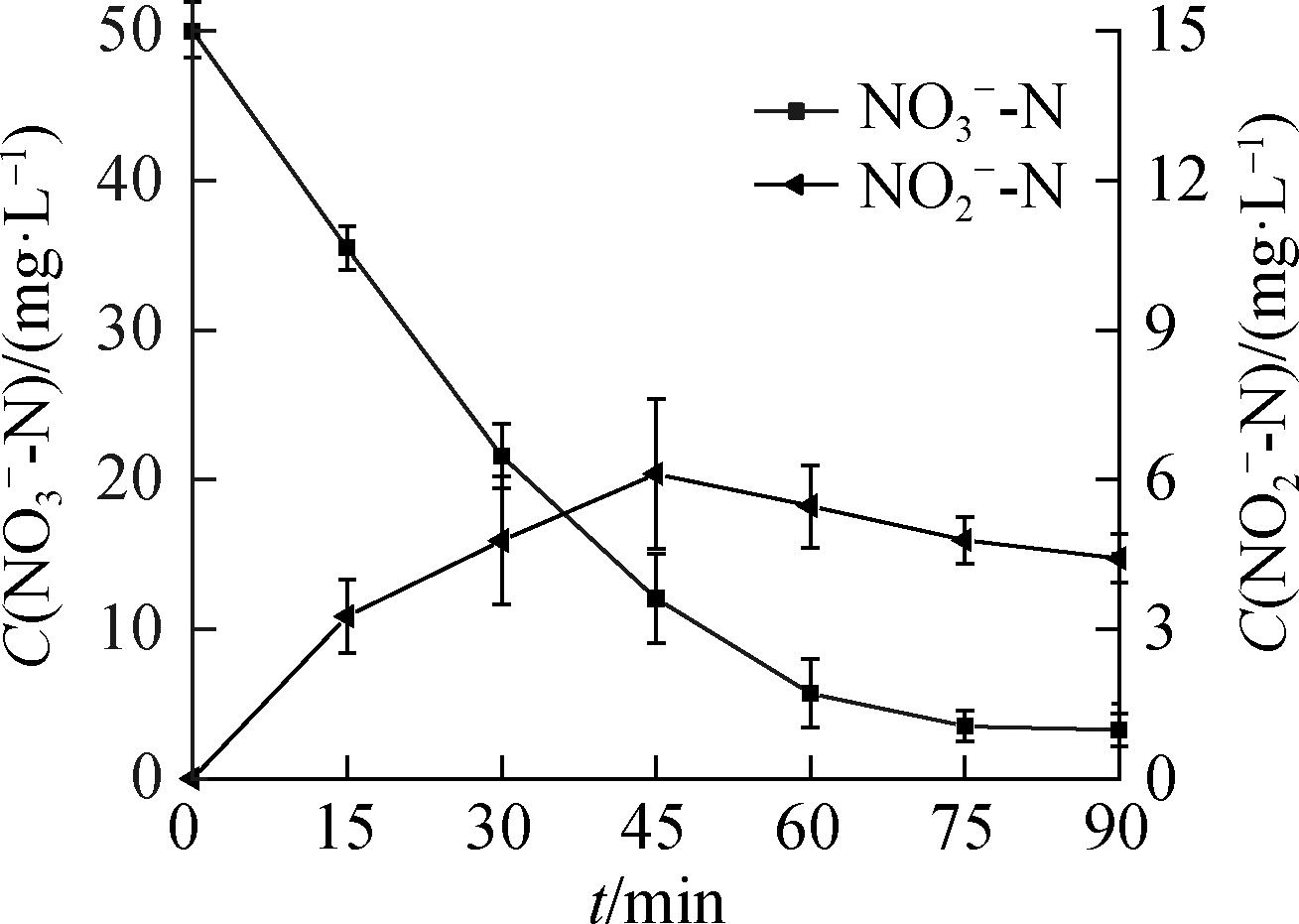
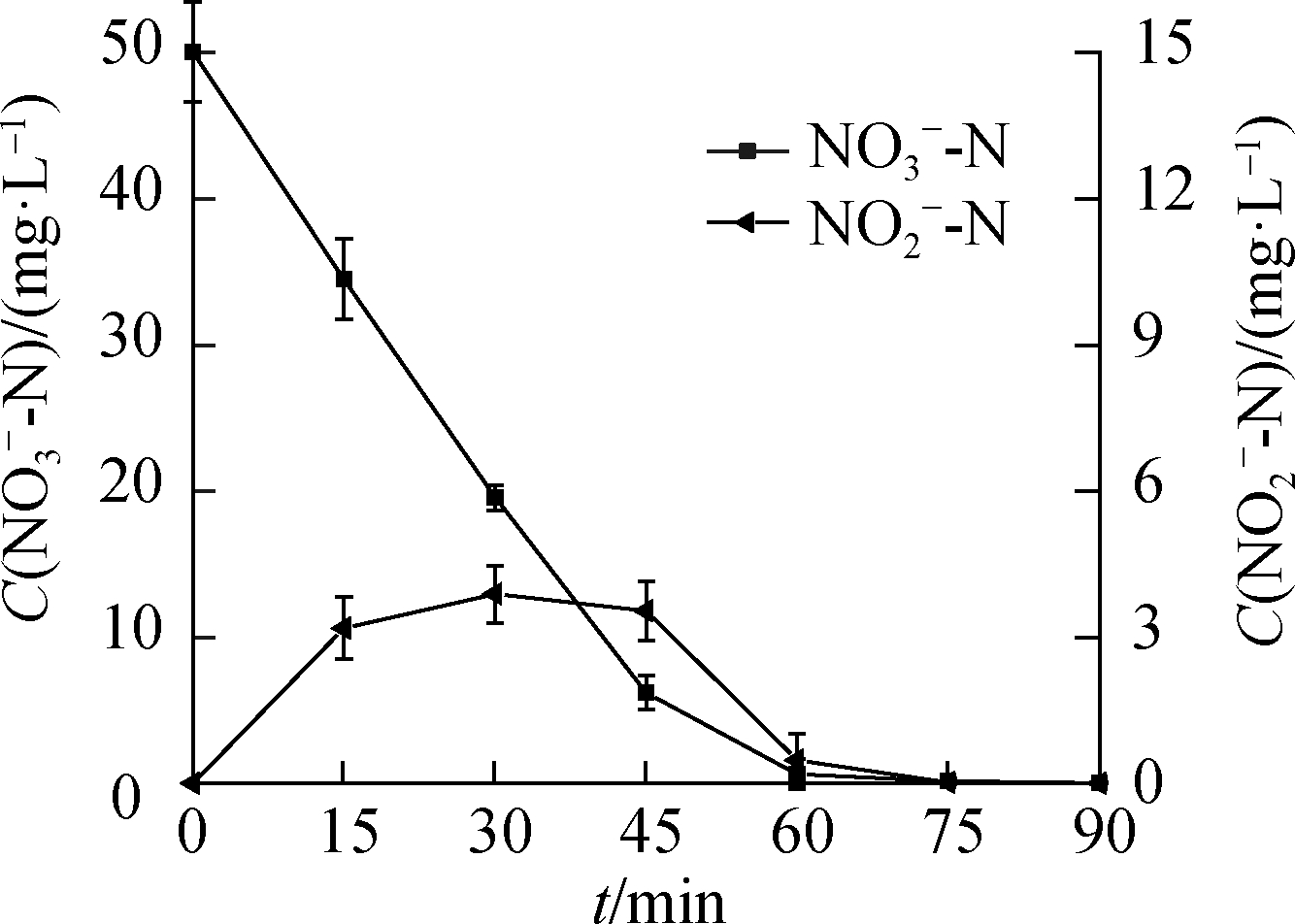
Fig.3 shows that ![]() accumulated during the
accumulated during the ![]() reduction process.As the C0(HCOOH)concentration increased, the concentration of
reduction process.As the C0(HCOOH)concentration increased, the concentration of ![]() gradually decreased at 90 min of the reaction, indicating that part of
gradually decreased at 90 min of the reaction, indicating that part of ![]() participated in the reduction reaction to produce
participated in the reduction reaction to produce ![]() and was further reduced.
and was further reduced.![]() production showed a trend of first increasing and then decreasing, except at C0(HCOOH)=3 mmol/L, peaking at 60, 45, and 30 min.
production showed a trend of first increasing and then decreasing, except at C0(HCOOH)=3 mmol/L, peaking at 60, 45, and 30 min.

(a)
(b)
(c)
(d)
Fig.3 ![]() reduction.(a)C0(HCOOH)=3 mmol/L;(b)C0(HCOOH)=5 mmol/L;(c)C0(HCOOH)=7 mmol/L;(d)C0(HCOOH)=9 mmol/L
reduction.(a)C0(HCOOH)=3 mmol/L;(b)C0(HCOOH)=5 mmol/L;(c)C0(HCOOH)=7 mmol/L;(d)C0(HCOOH)=9 mmol/L
With ![]() mg/L and C0(HCOOH)=9 mmol/L, the highest
mg/L and C0(HCOOH)=9 mmol/L, the highest ![]() concentration(i.e., 3.9 mg/L)was achieved at 30 min.As the reaction proceeded,
concentration(i.e., 3.9 mg/L)was achieved at 30 min.As the reaction proceeded, ![]() began to degrade, and its concentration decreased accordingly.Complete
began to degrade, and its concentration decreased accordingly.Complete ![]() degradation was reached at 90 min.Consequently,
degradation was reached at 90 min.Consequently, ![]() reduction by UV/HCOOH/N2 ARP did not induce
reduction by UV/HCOOH/N2 ARP did not induce ![]() pollution.
pollution.
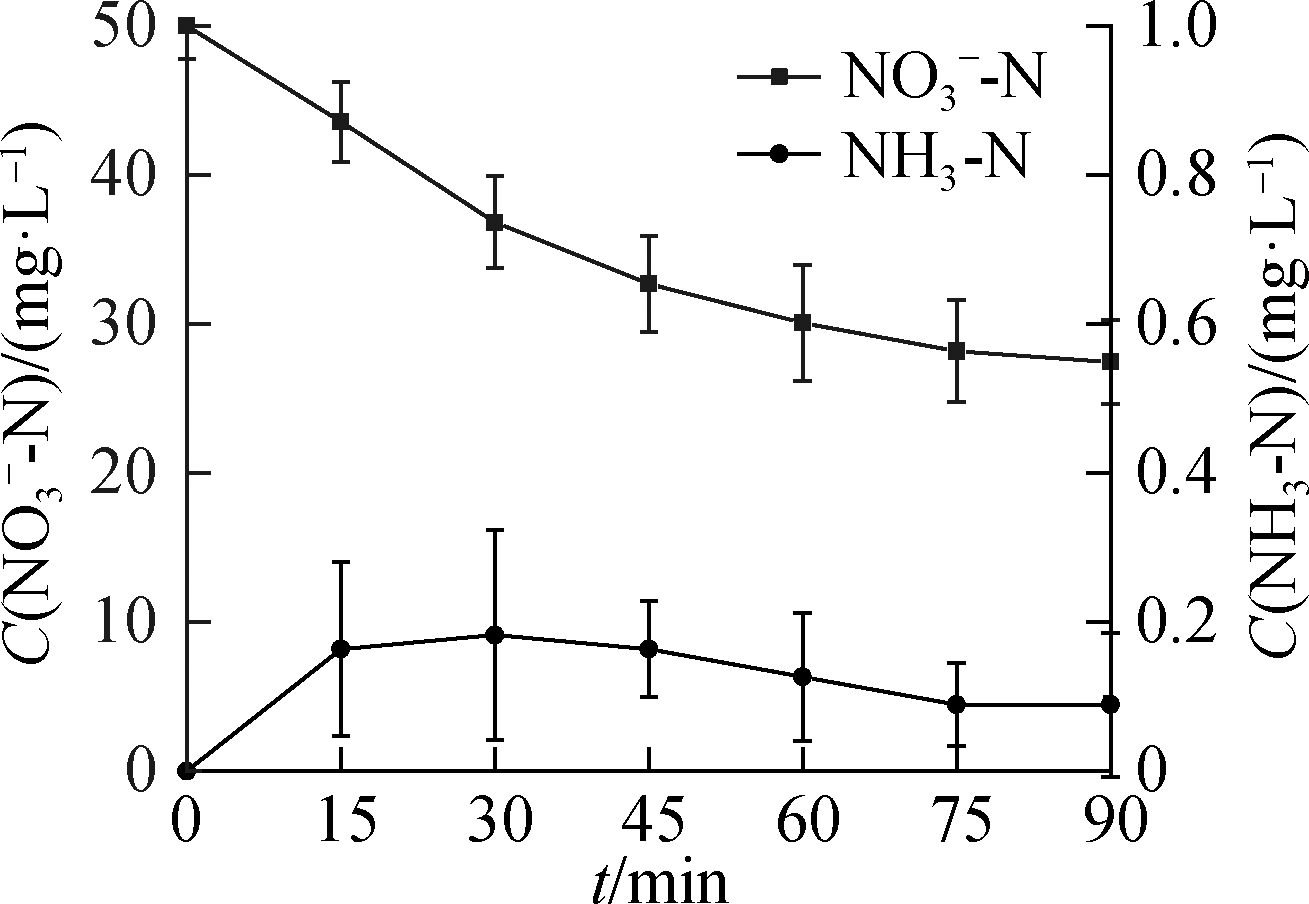
NH3-N generation is shown in Fig.4.HCOOH dosage had a certain influence on the amount of NH3-N.However, in general, NH3-N concentration remained at a low level and tended to reach a certain value.This finding indicates that NH3 is irreversible in the ![]() reduction process, thus determining the final denitrification and gas conversion efficiencies.When the C0(HCOOH)concentration was 9 mM, the NH3 concentration was always less than 0.3 mg/L.At 90 min, when the degradation of
reduction process, thus determining the final denitrification and gas conversion efficiencies.When the C0(HCOOH)concentration was 9 mM, the NH3 concentration was always less than 0.3 mg/L.At 90 min, when the degradation of ![]() was completed, NH3-N production was less than 0.6%.
was completed, NH3-N production was less than 0.6%.
(a)
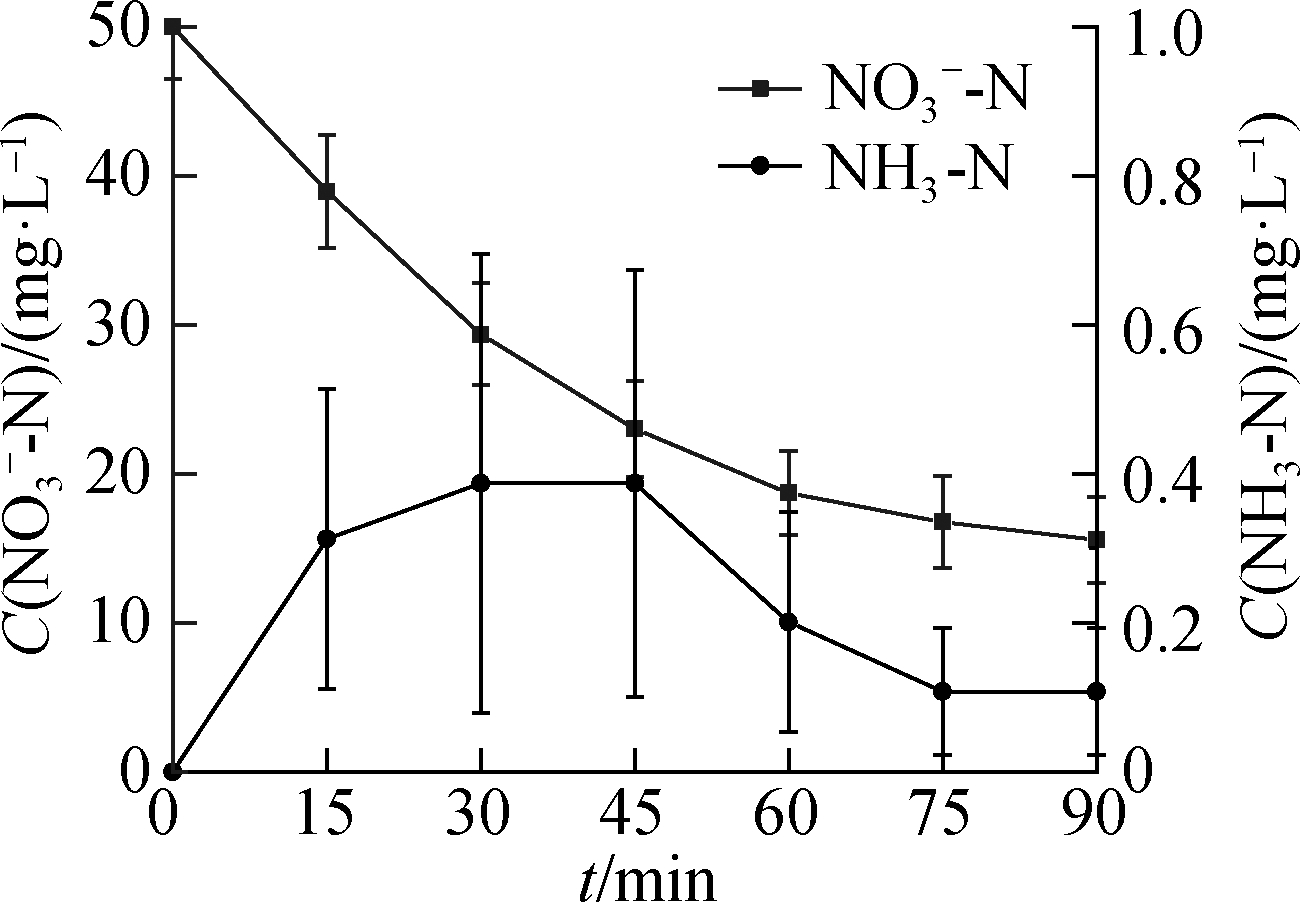
(b)
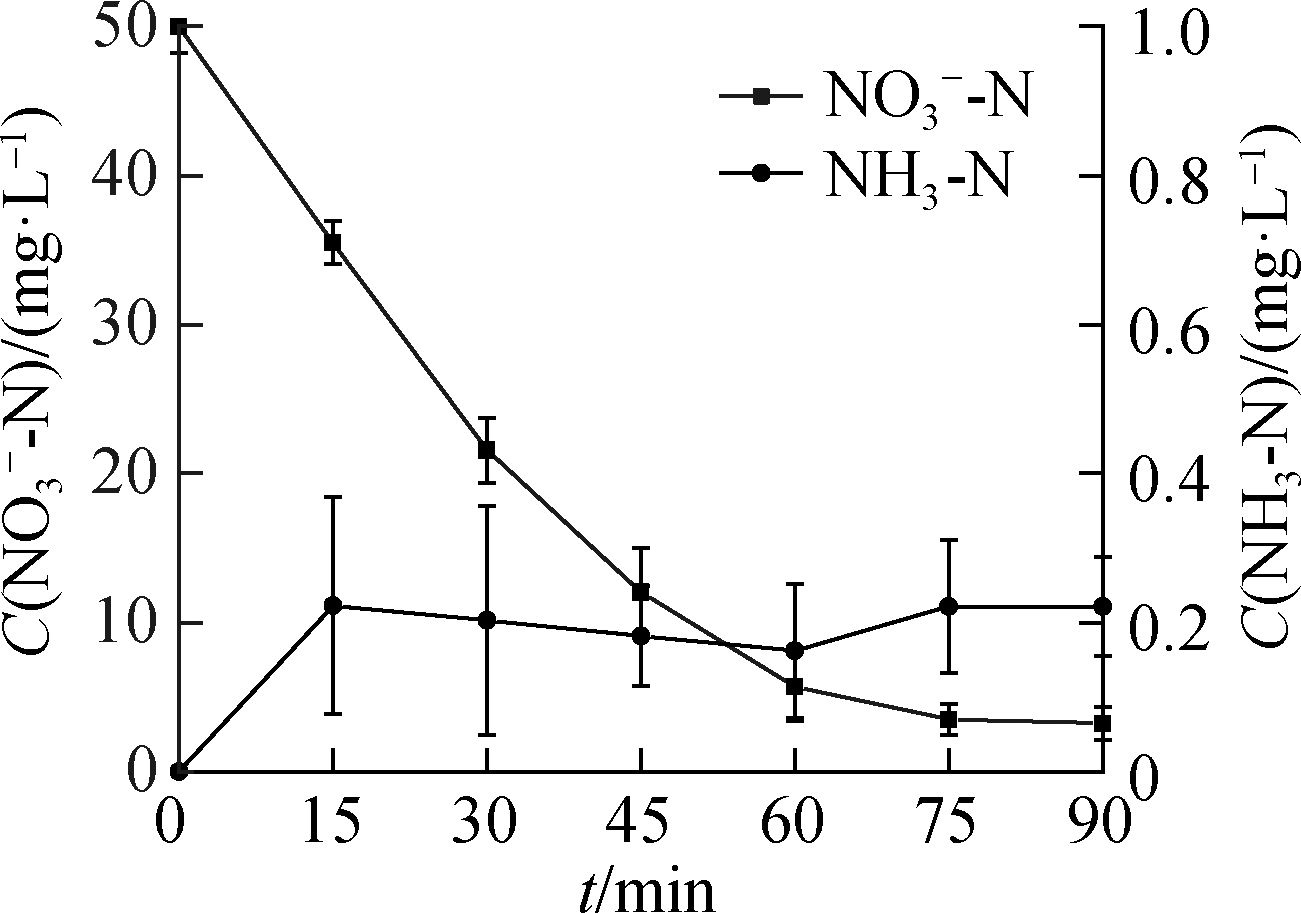
(c)
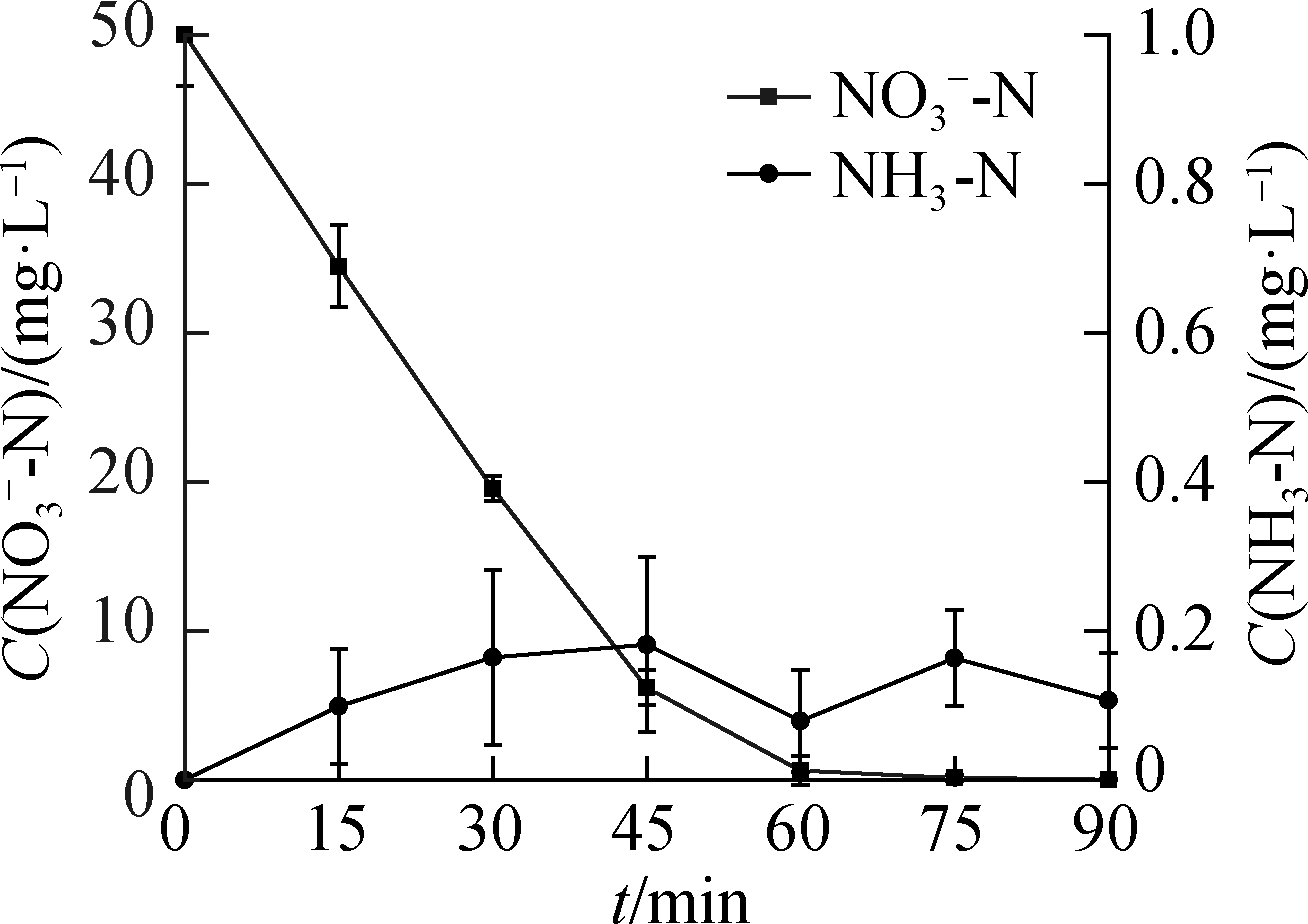
(d)
Fig.4 NH3-N formation from ![]() reduction.(a)C0(HCOOH)=3 mmol/L;(b)C0(HCOOH)=5 mmol/L;(c)C0(HCOOH)=7 mmol/L;(d)C0(HCOOH)=9 mmol/L
reduction.(a)C0(HCOOH)=3 mmol/L;(b)C0(HCOOH)=5 mmol/L;(c)C0(HCOOH)=7 mmol/L;(d)C0(HCOOH)=9 mmol/L
![]() was replaced with
was replaced with ![]() of equal molar concentration for further mechanism analysis.Under 9 mM of HCOOH, the reduction rate of
of equal molar concentration for further mechanism analysis.Under 9 mM of HCOOH, the reduction rate of ![]() was higher than that of
was higher than that of ![]() (nearly complete within 30 min), but more by-products were generated.As shown in Fig.5(a), the decrease in the concentration of
(nearly complete within 30 min), but more by-products were generated.As shown in Fig.5(a), the decrease in the concentration of ![]() was accompanied by the formation of NH3, the concentration of which reached the highest value(i.e., 4.2 mg/L)at 45 min and tended to maintain a constant value(i.e., 3.1 mg/L)at the end of the reaction.The production and accumulation of NH3 indicated that
was accompanied by the formation of NH3, the concentration of which reached the highest value(i.e., 4.2 mg/L)at 45 min and tended to maintain a constant value(i.e., 3.1 mg/L)at the end of the reaction.The production and accumulation of NH3 indicated that ![]() was not the only product of the first conversion of
was not the only product of the first conversion of ![]() reduction by UV-activated HCOOH.The direct conversion of
reduction by UV-activated HCOOH.The direct conversion of ![]() into gas also occurred.
into gas also occurred.
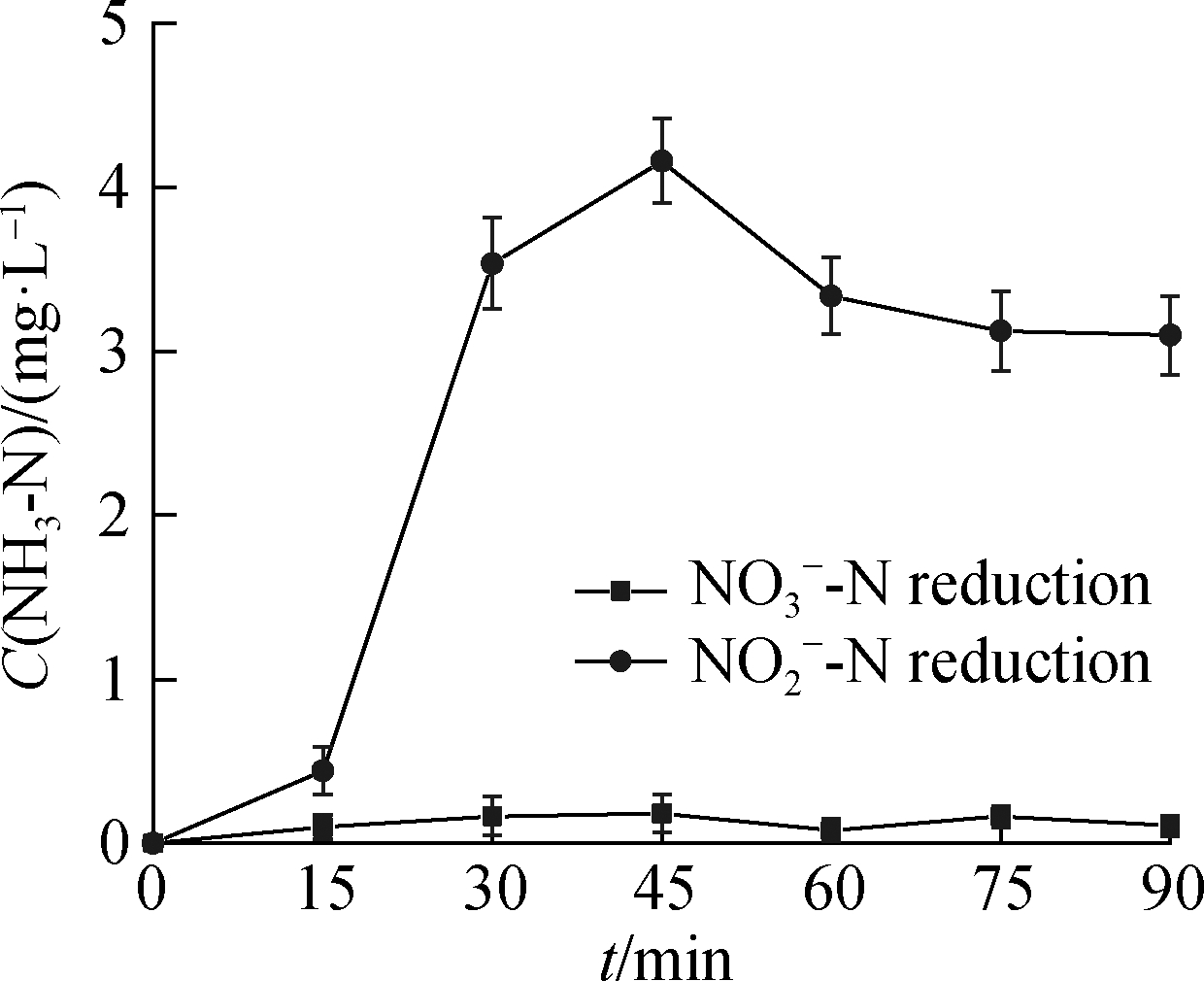
(a)
(b)
Fig.5 ![]() reduction by UV/HCOOH.(a)NH3-N formation compared with
reduction by UV/HCOOH.(a)NH3-N formation compared with ![]() reduction products
reduction products
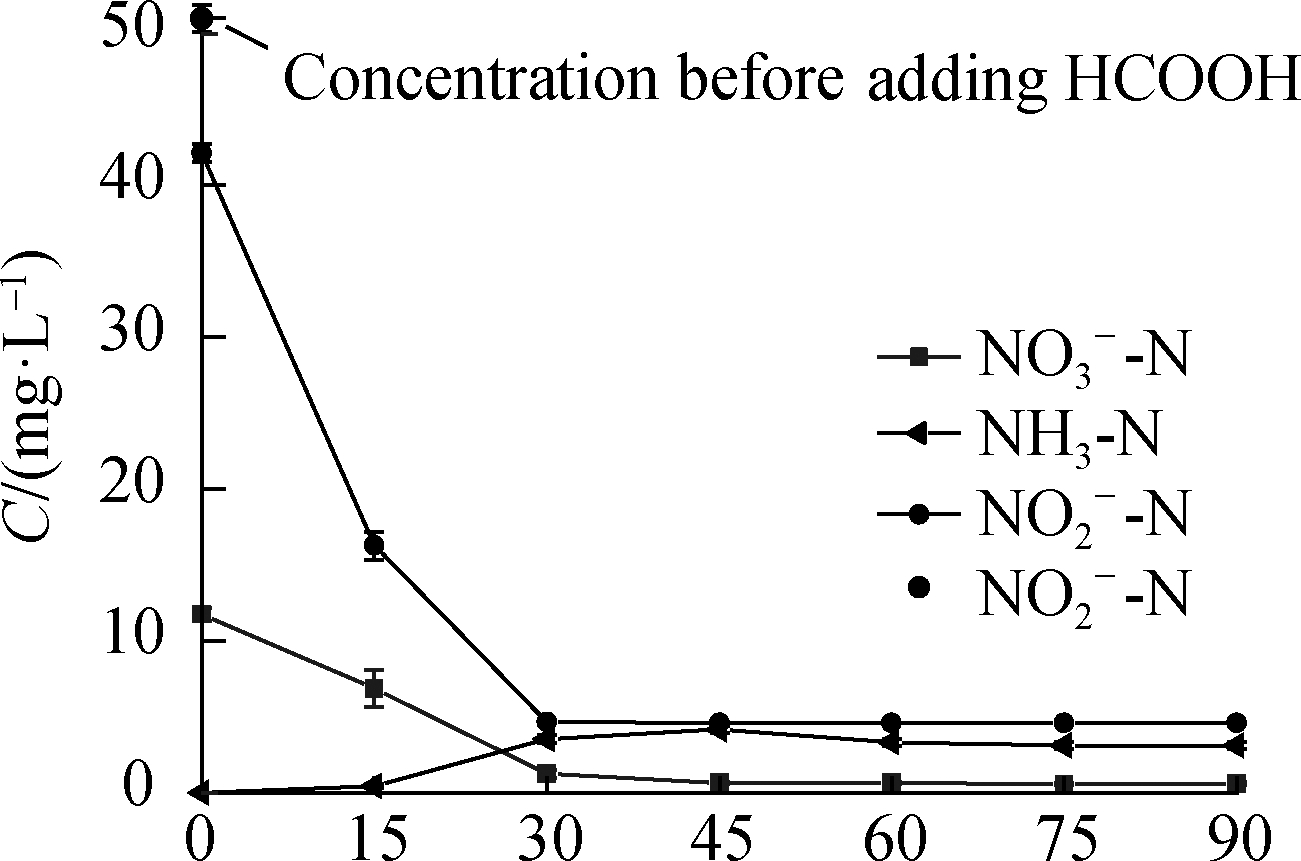
Fig.5(b)illustrates the existence of a certain amount of ![]() before the reaction started, and the
before the reaction started, and the ![]() concentration gradually decreased as the reaction proceeded.The
concentration gradually decreased as the reaction proceeded.The ![]() concentration decreased by approximately 20% after the addition of HCOOH, and the sum of this concentration and the
concentration decreased by approximately 20% after the addition of HCOOH, and the sum of this concentration and the ![]() concentration was nearly equal to the concentration of
concentration was nearly equal to the concentration of ![]() in the solution before the addition of HCOOH.The possible reason is that the solution became acidic after the addition of HCOOH, and
in the solution before the addition of HCOOH.The possible reason is that the solution became acidic after the addition of HCOOH, and ![]() tended to decompose under acidic conditions, producing
tended to decompose under acidic conditions, producing ![]() .To confirm this finding, a parallel experiment was conducted to replace HCOOH with HCl, the pH was adjusted to 3.0, and a similar concentration ratio of
.To confirm this finding, a parallel experiment was conducted to replace HCOOH with HCl, the pH was adjusted to 3.0, and a similar concentration ratio of ![]() and
and ![]() was observed.
was observed.
HCOOH, ![]() and HCOOH/C3H8O were subjected to in situ illumination and characterized by EPR for radical detection.HCOOH alone and
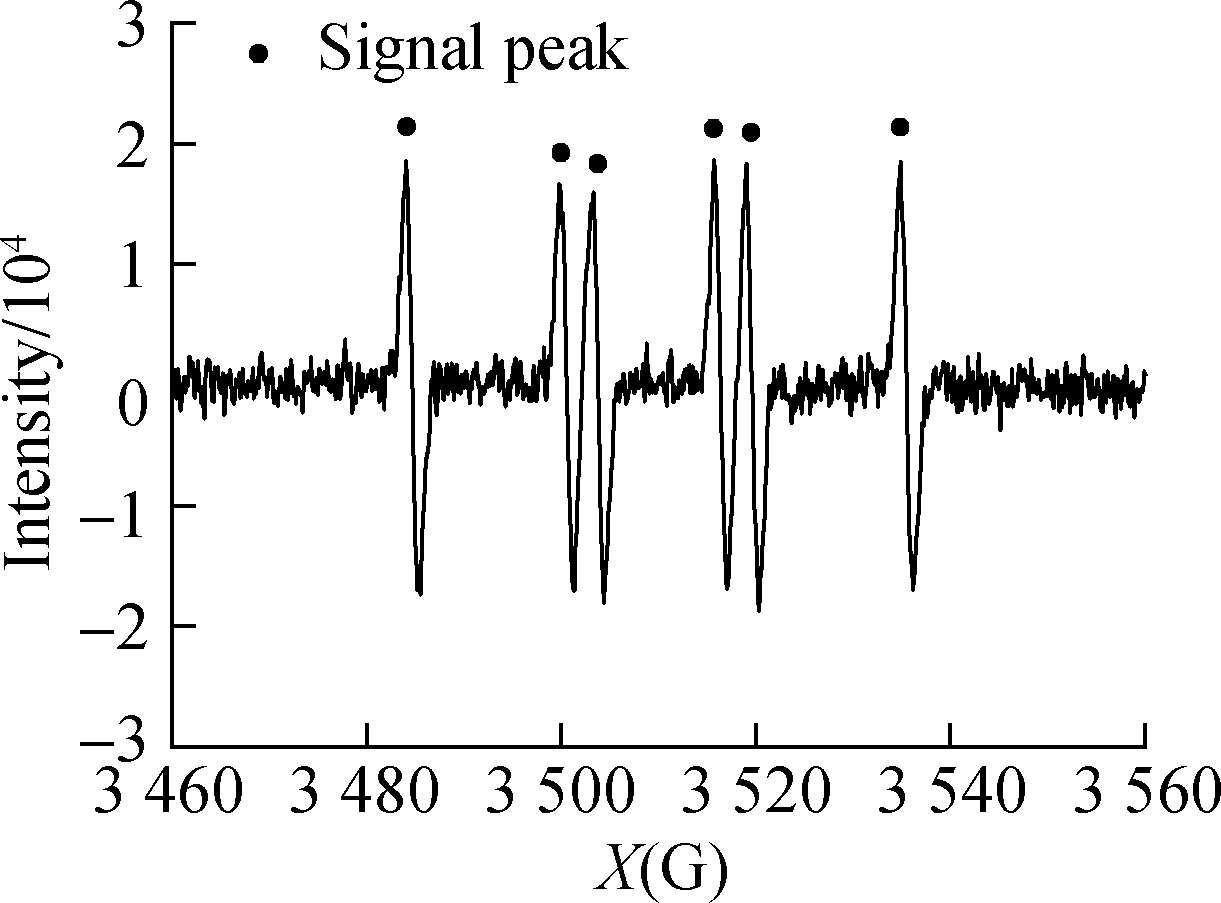
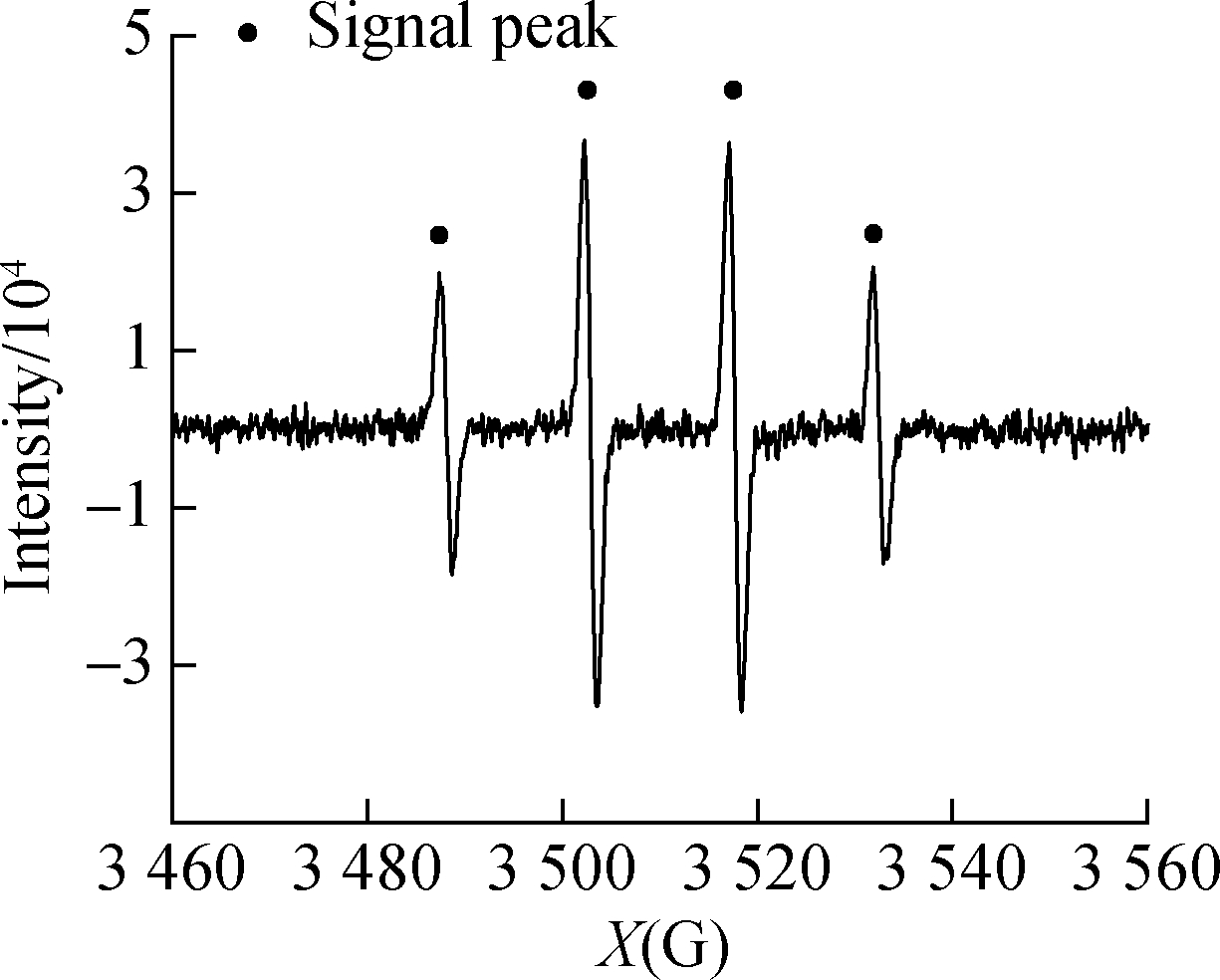
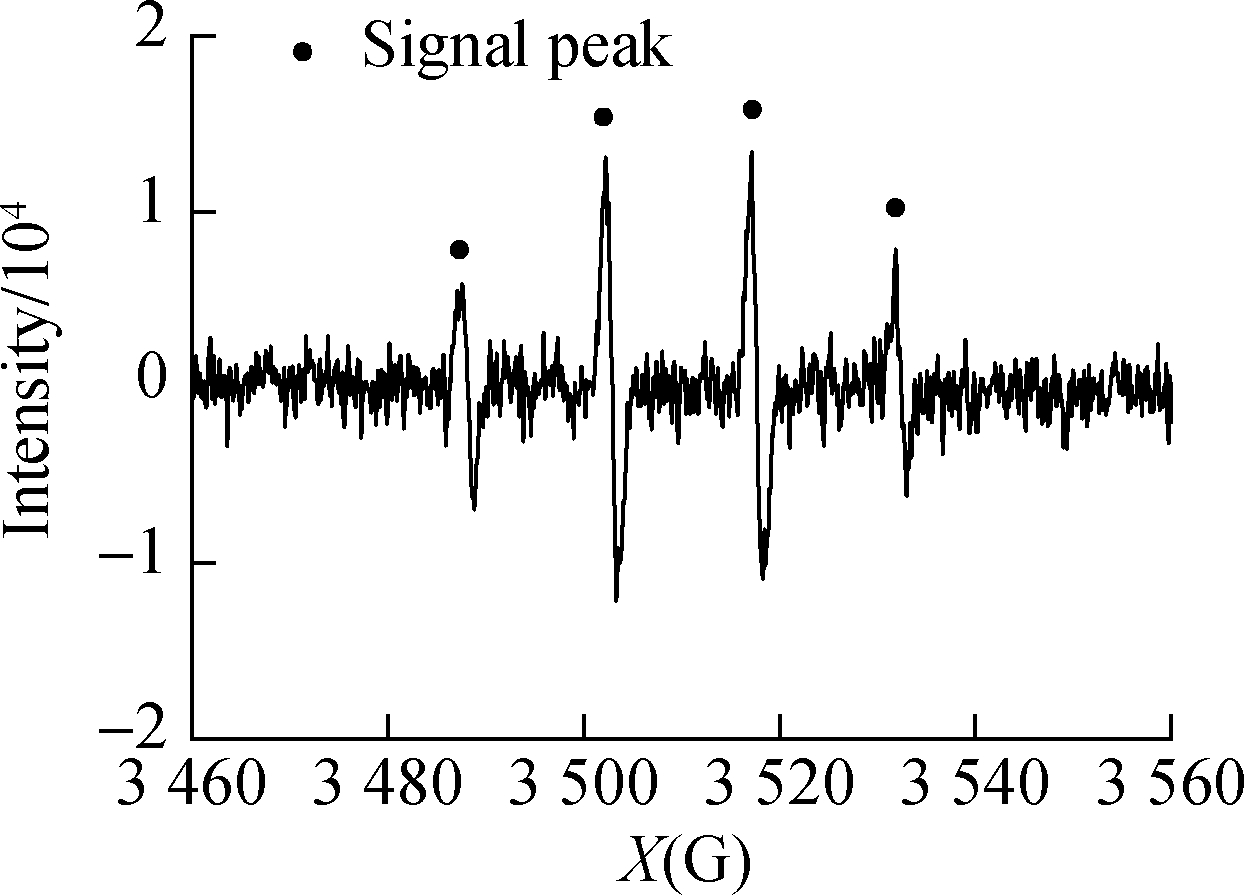
and HCOOH/C3H8O were subjected to in situ illumination and characterized by EPR for radical detection.HCOOH alone and ![]() showed no signal peak in darkness(see Figs.6(a)and(b)).After UV illumination, the signal peak(m(H)=19.1 g, m(N)=15.8 g)of DMPO-·
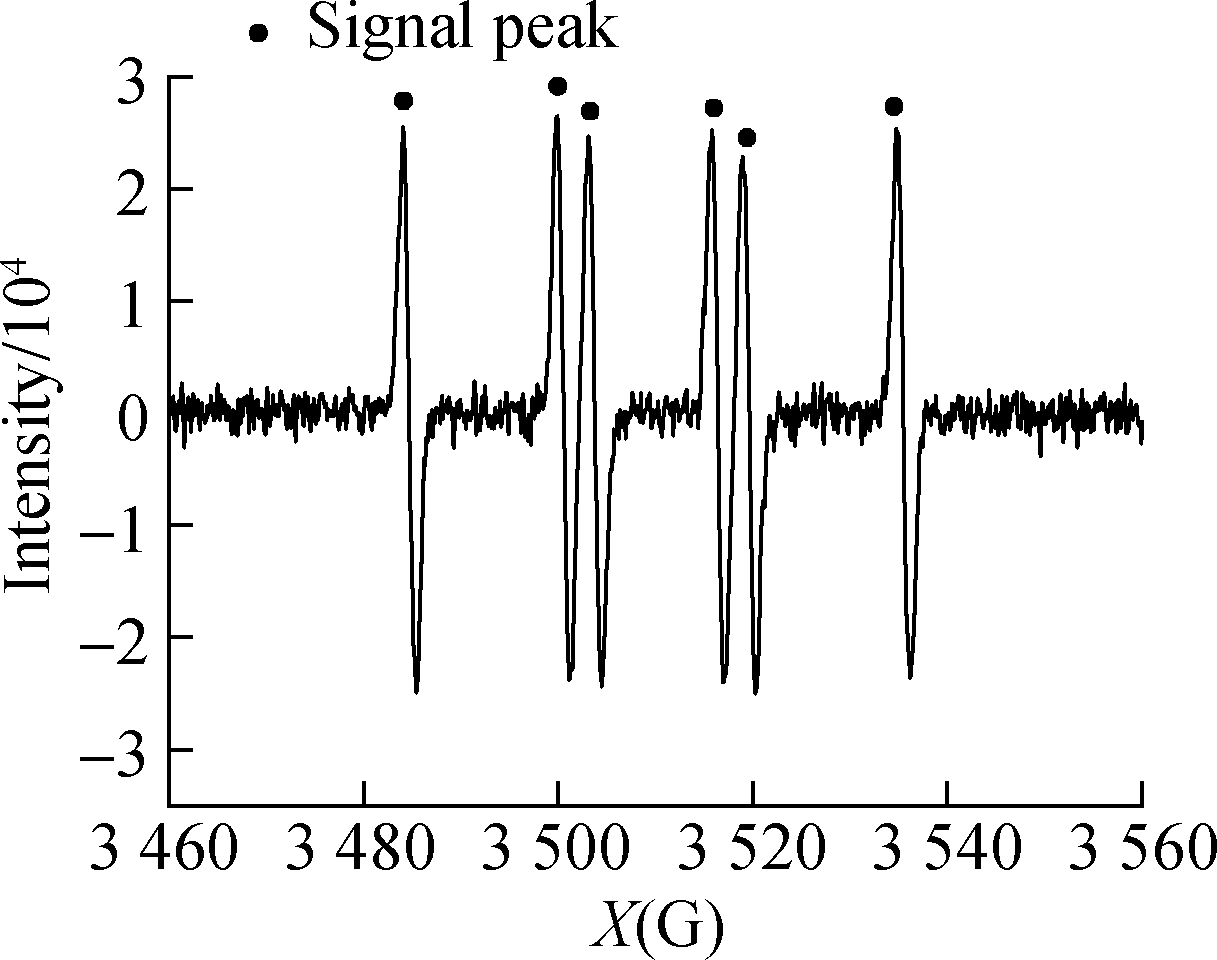
showed no signal peak in darkness(see Figs.6(a)and(b)).After UV illumination, the signal peak(m(H)=19.1 g, m(N)=15.8 g)of DMPO-·![]() appeared in both systems(see Figs.6(c)and(d)), confirming the generation of·
appeared in both systems(see Figs.6(c)and(d)), confirming the generation of·![]() .The peak intensity of the
.The peak intensity of the ![]() system was low because of the reaction between·
system was low because of the reaction between·![]() Figs.6(e)and(f)show the EPR detection of hydroxyl radical(·OH)from UV/HCOOH/C3H8O and HCOOH/C3H8O, respectively.A signal peak with the strength of 1:2:2:1 appeared in the UV/HCOOH system, proving the existence of·OH[20-21].After the addition of isopropanol as·OH quencher, the signal peak of·OH weakened evidently because of the consumption of radicals by the quencher.This finding coincides with that reported by Harbour et al.[19], which confirmed that·
Figs.6(e)and(f)show the EPR detection of hydroxyl radical(·OH)from UV/HCOOH/C3H8O and HCOOH/C3H8O, respectively.A signal peak with the strength of 1:2:2:1 appeared in the UV/HCOOH system, proving the existence of·OH[20-21].After the addition of isopropanol as·OH quencher, the signal peak of·OH weakened evidently because of the consumption of radicals by the quencher.This finding coincides with that reported by Harbour et al.[19], which confirmed that·![]() generation was caused by·OH.
generation was caused by·OH.
(a)
(b)
(c)
(d)
(e)
(f)
Fig.6 EPR spectra of radicals.(a)DMPO-·![]() from UV/HCOOH;(b)DMPO-·
from UV/HCOOH;(b)DMPO-·![]() (c)DMPO-·OH from UV/HCOOH;(d)DMPO-·OH from UV/HCOOH/C3H8O;(e)HCOOH;(f)
(c)DMPO-·OH from UV/HCOOH;(d)DMPO-·OH from UV/HCOOH/C3H8O;(e)HCOOH;(f)![]()
From the previously presented discussion, the reaction paths of ![]() reduction by UV-activated HCOOH are proposed in Fig.7.
reduction by UV-activated HCOOH are proposed in Fig.7.
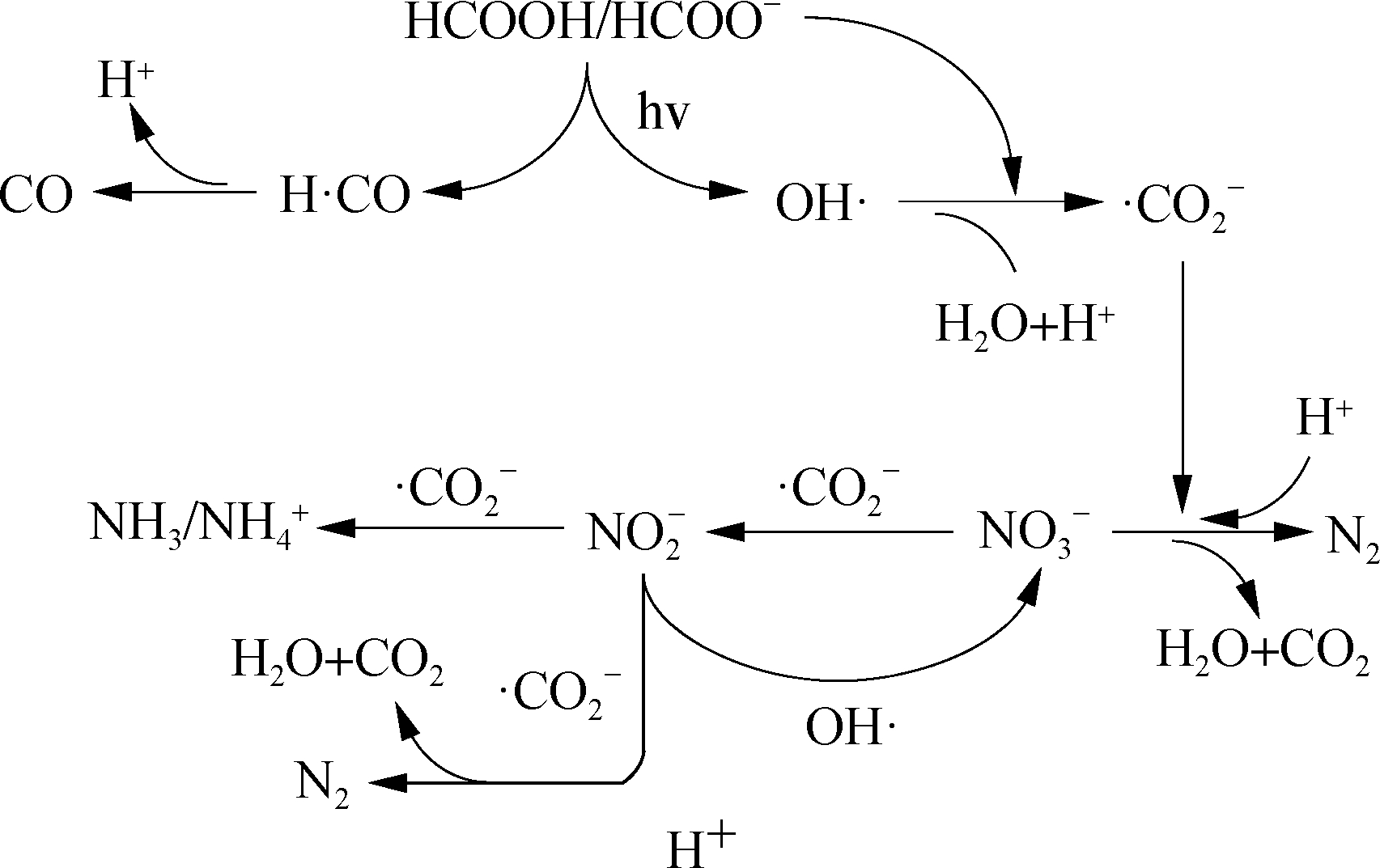
Fig.7 Conceptual reaction mechanism of UV-activated HCOOH denitrification
High-pressure mercury lamps with light intensities of 125, 175, and 250 W were selected to investigate the effect of light intensity.After 45 min of irradiation, the reduction ratios of ![]() at 125, 175, and 250 W were 88.2%, 97.8%, and 99.0%, respectively.This finding can be attributed to the enhancement of light intensity that accelerates the photon excitation rate of HCOOH to produce·OH, thus generating more·
at 125, 175, and 250 W were 88.2%, 97.8%, and 99.0%, respectively.This finding can be attributed to the enhancement of light intensity that accelerates the photon excitation rate of HCOOH to produce·OH, thus generating more·![]() .At the end of the reaction, the reduction rates of
.At the end of the reaction, the reduction rates of ![]() at three light intensities all exceeded 99.9%.
at three light intensities all exceeded 99.9%.
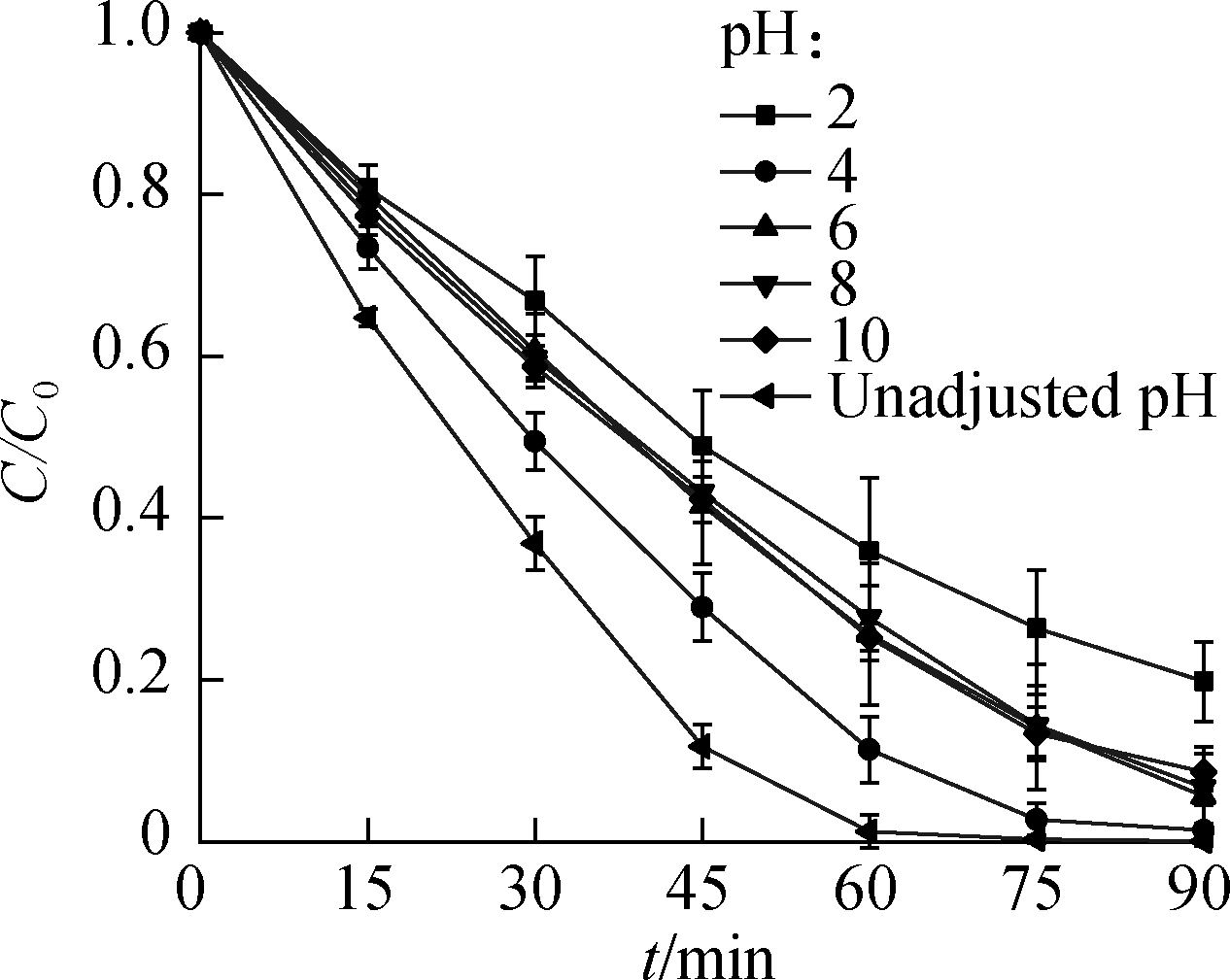
Six pH gradients, i.e., 2.0, 4.0, 6.0, 8.0, 10.0, and unadjusted pH(i.e., 3.0), were applied to verify the effect of pH on·![]() denitrification.Fig.8 illustrates that, at 90 min, the
denitrification.Fig.8 illustrates that, at 90 min, the ![]() reduction ratio(i.e., 99.9%)and gas conversion ratio(i.e., 99.8%)both peaked under unadjusted pH(i.e., 3.0).When the pH increased to 4.0, the reduction and gas conversion ratios of
reduction ratio(i.e., 99.9%)and gas conversion ratio(i.e., 99.8%)both peaked under unadjusted pH(i.e., 3.0).When the pH increased to 4.0, the reduction and gas conversion ratios of ![]() still reached high levels at the end of the reaction(i.e., 98.6% and 98.2%, respectively)with a slight decrease in the reduction rate.When the initial pH successively increased to 6.0, 8.0, and 10.0, the reduction efficiency and gas conversion ratio of
still reached high levels at the end of the reaction(i.e., 98.6% and 98.2%, respectively)with a slight decrease in the reduction rate.When the initial pH successively increased to 6.0, 8.0, and 10.0, the reduction efficiency and gas conversion ratio of ![]() remarkably decreased.Given that pKa(HCOOH)= 3.75 when pH > 3.75, the main existing form of HCOOH is HCOO-, whose ability to produce·
remarkably decreased.Given that pKa(HCOOH)= 3.75 when pH > 3.75, the main existing form of HCOOH is HCOO-, whose ability to produce·![]() under UV irradiation is weak, thus inhibiting the reduction of
under UV irradiation is weak, thus inhibiting the reduction of ![]() .At pH=2.0, the removal and gas conversion ratios of
.At pH=2.0, the removal and gas conversion ratios of ![]() after 90 min were the lowest, i.e., 80.2% and 69.2%, respectively.The decomposition of REDOX-active groups in the solution under a hyperacid environment can explain the decrease in the reduction effect.
after 90 min were the lowest, i.e., 80.2% and 69.2%, respectively.The decomposition of REDOX-active groups in the solution under a hyperacid environment can explain the decrease in the reduction effect.
(a)

(b)
Fig.8 Effects of pH on ![]() reduction.(a)Reduction ratio;(b)Gas conversion ratio
reduction.(a)Reduction ratio;(b)Gas conversion ratio
Solution pH variation is shown in Fig.9.When the initial pH was 2.0 and 10.0, the solution pH remained unchanged within 90 min.When the initial pH was 4.0, 6.0, 8.0, and unadjusted(i.e., 3.0), the solution pH increased with time and reached a certain value eventually, indicating that a large amount of HCOOH was consumed in the UV activation process, resulting in the solution pH changing to neutral and weakly alkaline.
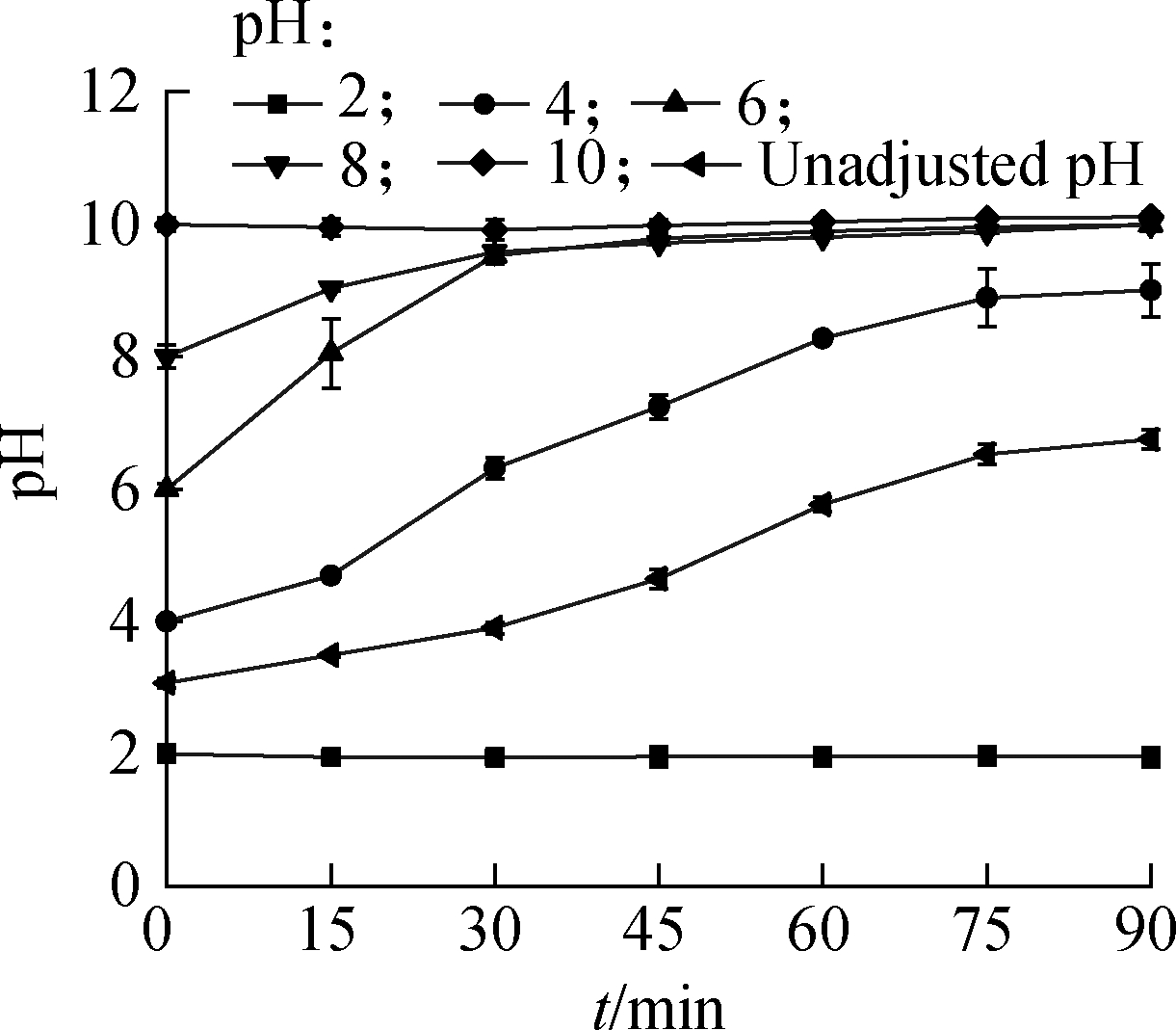
Fig.9 pH variation with reaction time
Consequently,the initial pH is one of the main factors that influence the UV/HCOOH/N2 denitrification system.The optimal pH is 3 to 4.Meanwhile, a hyperacid environment has an adverse effect on the reduction effect, and a neutral or alkaline environment also reduces the reduction effect but to a slight extent.
The influence of common anions in natural water wasinvestigated under a 125 W high-pressure mercury lamp.The concentration of anions was set at 1, 10, and 100 mmol/L.
Cl- and ![]() reduction.As the initial anion concentration increased, the reduction rates slightly decreased.With Cl- or
reduction.As the initial anion concentration increased, the reduction rates slightly decreased.With Cl- or ![]() could still reach 98.0% and 98.9%, respectively.
could still reach 98.0% and 98.9%, respectively.
Fig.10 shows that, after 90 min, the removal ratio of ![]() showed only a slight change under 1 and 10 mM of
showed only a slight change under 1 and 10 mM of ![]() and a 3.5% decrease under 100 mM of
and a 3.5% decrease under 100 mM of ![]() .The addition of
.The addition of ![]() changed the initial pH to 3.27, 5.64, and 7.68.For the system with 100 mM of
changed the initial pH to 3.27, 5.64, and 7.68.For the system with 100 mM of ![]() , the initial pH was adjusted in the same way as when
, the initial pH was adjusted in the same way as when ![]() was not added.Moreover, the comparison confirms that, under acidic conditions,
was not added.Moreover, the comparison confirms that, under acidic conditions, ![]() reduction was less inhibited when
reduction was less inhibited when ![]() was converted into
was converted into ![]() .Therefore,
.Therefore, ![]() can inhibit
can inhibit ![]() reduction in two ways: 1)Under a high
reduction in two ways: 1)Under a high ![]() concentration, the increase in the initial pH of the solution inhibits the reduction of
concentration, the increase in the initial pH of the solution inhibits the reduction of ![]() 2)·OH yielded by UV-activated HCOOH reacts with
2)·OH yielded by UV-activated HCOOH reacts with ![]() blocking the production of·OH[22] as
blocking the production of·OH[22] as
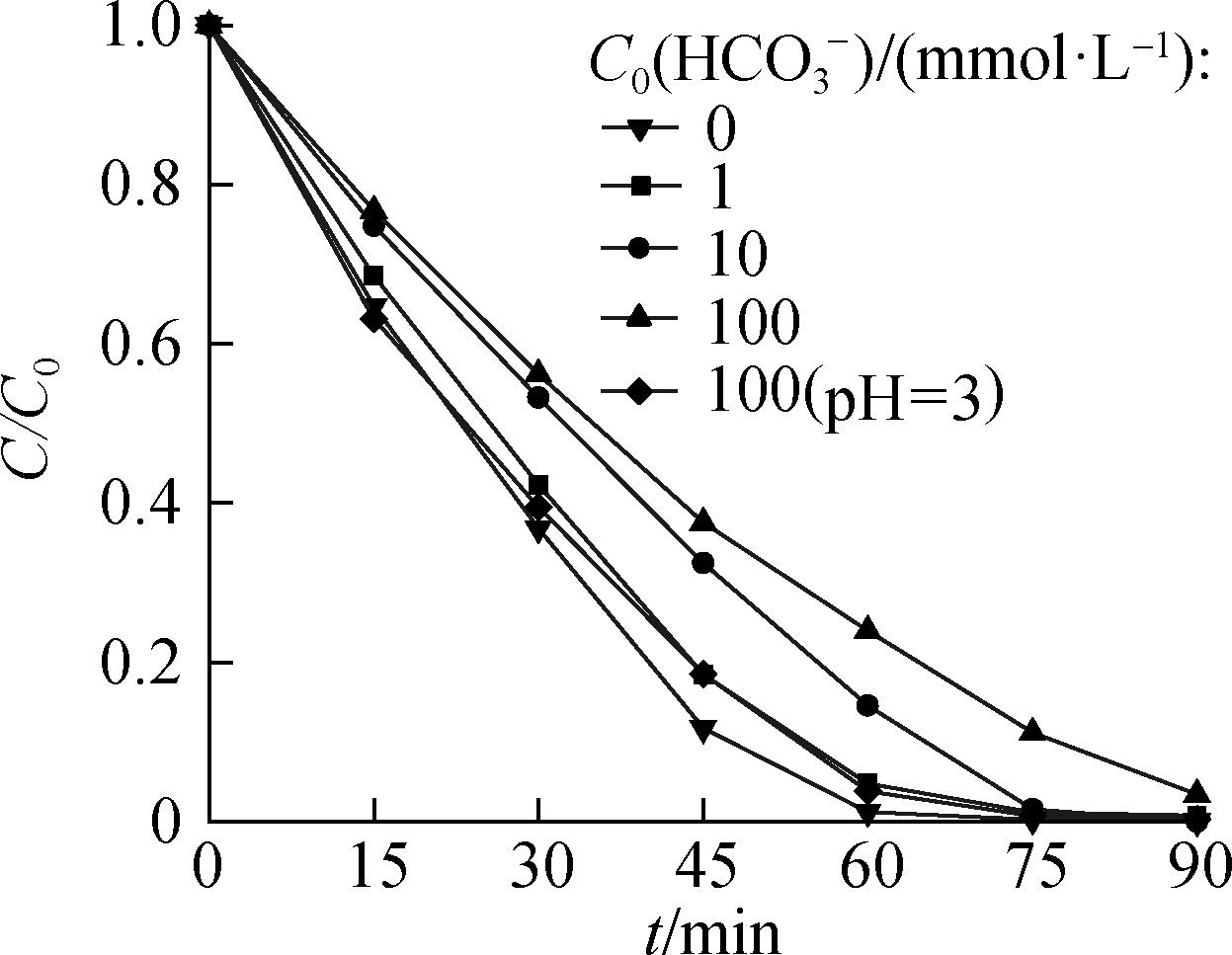
Fig.10 Effects of ![]() reduction
reduction
·![]()
(1)
Furthermore, when the concentration of ![]() further increases, it reacts with·
further increases, it reacts with·![]() as follows[23]:
as follows[23]:
·![]() ·
·![]()
Therefore, the presence of ![]() has a certain effect on the reduction of
has a certain effect on the reduction of ![]()
The kinetics of ![]() reduction by UV/HCOOH/N2 was analyzed for further interpretation.The sampling interval was reduced to 5 min, and the experiments were conducted with a thermostatic magnetic stirrer to stabilize the temperature at 25 ℃.
reduction by UV/HCOOH/N2 was analyzed for further interpretation.The sampling interval was reduced to 5 min, and the experiments were conducted with a thermostatic magnetic stirrer to stabilize the temperature at 25 ℃.
First, the reduction curve of ![]() under a 125 W mercury lamp with C0(HCOOH)=9 mmol/L was fitted with C/C0 and ln(C/C0)with ordinate and reaction time as abscissa.The entire process can be divided into two stages; namely, a zero-order kinetic reaction in the early stage and first-order kinetic reaction in the late stage.In the first 30 min, the reduction process conformed to the zero-order reaction kinetics, and the corresponding zero-order rate constant was 1.03 min-1.At the later stage, the
under a 125 W mercury lamp with C0(HCOOH)=9 mmol/L was fitted with C/C0 and ln(C/C0)with ordinate and reaction time as abscissa.The entire process can be divided into two stages; namely, a zero-order kinetic reaction in the early stage and first-order kinetic reaction in the late stage.In the first 30 min, the reduction process conformed to the zero-order reaction kinetics, and the corresponding zero-order rate constant was 1.03 min-1.At the later stage, the ![]() reduction process fitted the first-order reaction equation, and the corresponding rate constant was 0.08 min-1.The difference between the two stages was caused by the formation of
reduction process fitted the first-order reaction equation, and the corresponding rate constant was 0.08 min-1.The difference between the two stages was caused by the formation of ![]() reduction process.
reduction process.
The influence of C0(HCOOH), initial pH, and light intensity on the ![]() reduction kinetics was investigated.Due to the limitation of the
reduction kinetics was investigated.Due to the limitation of the ![]() removal ratio, only zero-order kinetic fitting was conducted for degradation under different light intensities.The kinetic parameters shown in Tab.1 indicate that C0(HCOOH)and light intensity are the main factors that influence the
removal ratio, only zero-order kinetic fitting was conducted for degradation under different light intensities.The kinetic parameters shown in Tab.1 indicate that C0(HCOOH)and light intensity are the main factors that influence the ![]() reduction rate.Meanwhile, the initial pH has a relatively minimal influence.The increase in C0(HCOOH)concentration from 1 to 9 mM increased k0 of the zero-order stage from 0.005 8 to 0.021 1 min-1 and k1 of the first-order stage by 0.11 min-1.When the light intensity increased to 175 and 250 W, k0 increased to 0.026 and 0.043 min-1, respectively.
reduction rate.Meanwhile, the initial pH has a relatively minimal influence.The increase in C0(HCOOH)concentration from 1 to 9 mM increased k0 of the zero-order stage from 0.005 8 to 0.021 1 min-1 and k1 of the first-order stage by 0.11 min-1.When the light intensity increased to 175 and 250 W, k0 increased to 0.026 and 0.043 min-1, respectively.
Tab.1 Kinetic parameters of ![]() reduction under different influencing factors
reduction under different influencing factors
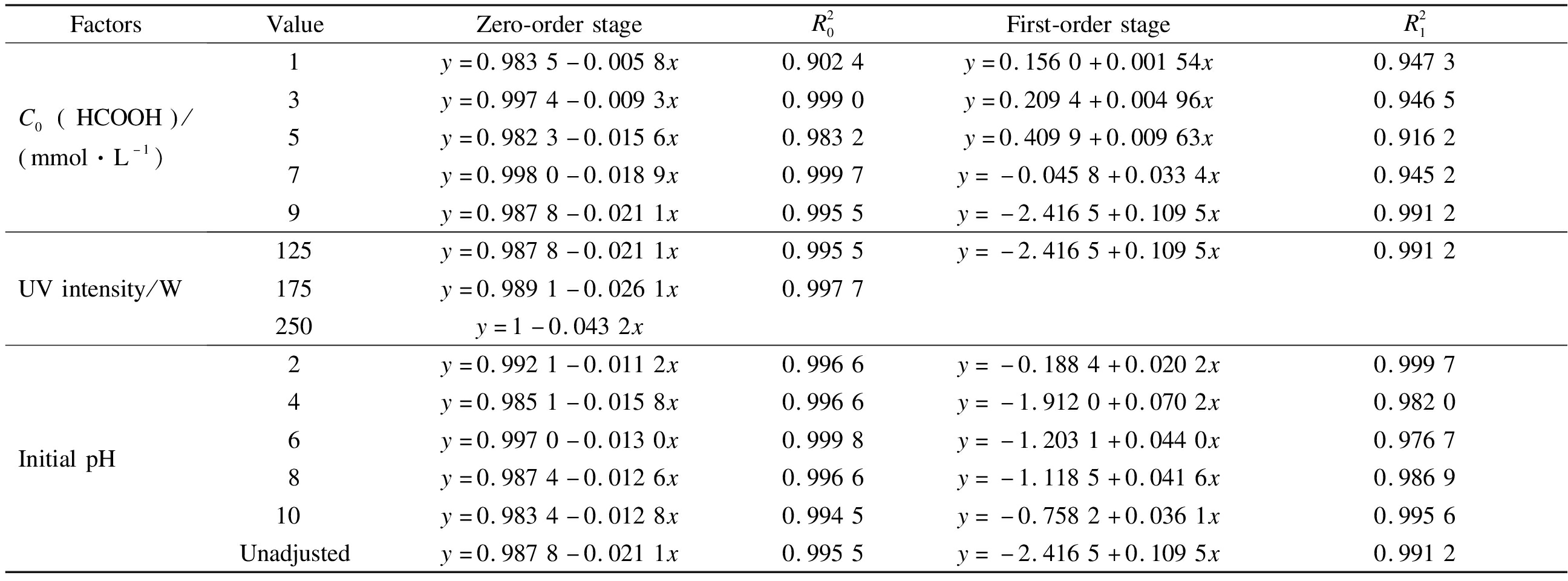
FactorsValueZero-orderstageR20First-orderstageR21C0 HCOOH / mmol·L-1)1y=0.9835-0.0058x0.9024y=0.1560+0.00154x0.94733y=0.9974-0.0093x0.9990y=0.2094+0.00496x0.94655y=0.9823-0.0156x0.9832y=0.4099+0.00963x0.91627y=0.9980-0.0189x0.9997y=-0.0458+0.0334x0.94529y=0.9878-0.0211x0.9955y=-2.4165+0.1095x0.9912UVintensity/W125y=0.9878-0.0211x0.9955y=-2.4165+0.1095x0.9912175y=0.9891-0.0261x0.9977250y=1-0.0432xInitialpH2y=0.9921-0.0112x0.9966y=-0.1884+0.0202x0.99974y=0.9851-0.0158x0.9966y=-1.9120+0.0702x0.98206y=0.9970-0.0130x0.9998y=-1.2031+0.0440x0.97678y=0.9874-0.0126x0.9966y=-1.1185+0.0416x0.986910y=0.9834-0.0128x0.9945y=-0.7582+0.0361x0.9956Unadjustedy=0.9878-0.0211x0.9955y=-2.4165+0.1095x0.9912
1)Under the basic conditions of 90 min of UV illumination, 9 mmol/L of C0(HCOOH), and N2 aeration, 99.9% of ![]() could be removed, and the gas conversion ratio of
could be removed, and the gas conversion ratio of ![]() could reach 99.8%.The residual HCOOH concentration was negligible(i.e.,0.3 mmol/L).
could reach 99.8%.The residual HCOOH concentration was negligible(i.e.,0.3 mmol/L).
2)![]() reduction by UV/HCOOH/N2 ARP was accompanied by the generation of
reduction by UV/HCOOH/N2 ARP was accompanied by the generation of ![]() and NH3 but did not cause secondary N pollution at the end of the reaction.
and NH3 but did not cause secondary N pollution at the end of the reaction.![]() was eventually reduced and removed from the solution, and the concentration of NH3 was always lower than 0.3 mg/L.The direct conversion of
was eventually reduced and removed from the solution, and the concentration of NH3 was always lower than 0.3 mg/L.The direct conversion of ![]() into gas occurred during the reaction process in addition to the formation of
into gas occurred during the reaction process in addition to the formation of ![]() and subsequent reduction to gas.
and subsequent reduction to gas.
3)EPR detection of in situ illumination proved that ![]() reduction was caused by·
reduction was caused by·![]() and·
and·![]() generation was caused by·OH.
generation was caused by·OH.
4)The initial HCOOH concentration, UV light intensity, and initial pH were the main factors that influenced the UV/HCOOH/N2 denitrification efficiency, and anions in natural water showed no significant effect on ![]() conversion.
conversion.
[1] Pacheco F A L, Santos R M B, Fernandes L F S, et al.Controls and forecasts of nitrate yields in forested watersheds: A view over mainland Portugal[J].Science of the Total Environment, 2015, 537: 421-440.DOI: 10.1016/j.scitotenv.2015.07.127.
[2] Huno S K M, Rene E R, Van Hullebusch E D, et al.Nitrate removal from groundwater: A review of natural and engineered processes[J].Journal of Water Supply: Research and Technology—Aqua, 2018, 67(8): 885-902.DOI: 10.2166/aqua.2018.194.
[3] Blarasin M, Cabrera A, Matiatos I, et al.Comparative evaluation of urban versus agricultural nitrate sources and sinks in an unconfined aquifer by isotopic and multivariate analyses[J].Science of the Total Environment, 2020, 741: 140374.DOI: 10.1016/j.scitotenv.2020.140374.
[4] Chen N W,Peng B R, Hong H S, et al.Nutrient enrichment and N:P ratio decline in a coastal bay-river system in southeast China: The need for a dual nutrient(N and P)management strategy[J].Ocean & Coastal Management, 2013, 81: 7-13.DOI: 10.1016/j.ocecoaman.2012.07.013.
[5] Dan-Hassan M A ,Olasehinde P I, Amadi A N, et al.Spatial and temporal distribution of nitrate pollution in groundwater of Abuja, Nigeria[J].International Journal of Chemistry, 2012, 4(3): 104-112.DOI: 10.5539/ijc.v4n3p104.
[6] Lotfata A, Ambinakudige S.Factors affecting the spatial pattern of nitrate contamination in Texas aquifers[J].Management of Environmental Quality: An International Journal, 2019, 31(4): 857-876.DOI: 10.1108/MEQ-05-2019-0097.
[7] Vystavna Y, Diadin D, Grynenko V, et al.Determination of dominant sources of nitrate contamination in transboundary(Russian Federation/Ukraine)catchment with heterogeneous land use[J].Environ Monit Assess, 2017, 189: 509.DOI: 10.1007/s10661-017-6227-5.
[8] Yan B Z, Xiao C L, Liang X J, et al.Impacts of urban land use on nitrate contamination in groundwater, Jilin City, Northeast China[J].Arabian Journal of Geosciences, 2016, 9(2): 105.DOI: 10.1007/s12517-015-2052-8.
[9] Liu Y, Wang J L.Reduction of nitrate by zero valent iron(ZVI)-based materials: A review[J].Sci Total Environ, 2019, 671: 388-403.DOI: 10.1016/j.scitotenv.2019.03.317.
[10] Wang B Q, An B H, Liu Y, et al.Selective reduction of nitrate into nitrogen at neutral pH range by iron/copper bimetal coupled with formate/ferric ion and ultraviolet radiation[J].Separation and Purification Technology, 2020, 248: 117061.DOI: 10.1016/j.seppur.2020.117061.
[11] Liu H, Liu X Y, Yang W W, et al.Photocatalytic dehydrogenation of formic acid promoted by a superior PdAg@g-C3N4 Mott-Schottky heterojunction[J].Journal of Materials Chemistry A, 2019, 7(5): 2022-2026.DOI: 10.1039/c8ta11172c.
[12] Jung B, Safan A, Duan Y, et al.Removal of arsenite by reductive precipitation in dithionite solution activated by UV light[J].Journal of Environmental Sciences, 2018, 74: 168-176.DOI: 10.1016/j.jes.2018.02.023.
[13] Xiao Q, Wang T, Yu S L, et al.Influence of UV lamp, sulfur(IV)concentration, and pH on bromate degradation in UV/sulfite systems: Mechanisms and applications[J].Water Research, 2017, 111: 288-296.DOI: 10.1016/j.watres.2017.01.018.
[14] Bensalah N, Nicola R, Abdel-Wahab A.Nitrate removal from water using ![]() advanced reduction process[J].International Journal of Environmental Science and Technology, 2013, 11(6): 1733-1742.DOI: 10.1007/s13762-013-0375-0.
advanced reduction process[J].International Journal of Environmental Science and Technology, 2013, 11(6): 1733-1742.DOI: 10.1007/s13762-013-0375-0.
[15] An B H, He H N, Duan B H, et al.Selective reduction of nitrite to nitrogen gas by CO2 anion radical from the activation of oxalate[J].Chemosphere, 2021, 278: 130388.DOI: 10.1016/j.chemosphere.2021.130388.
[16] Tugaoen H O, Garcia-Segura S, Hristovski K, et al.Challenges in photocatalytic reduction of nitrate as a water treatment technology[J].Science of the Total Environment, 2017, 599-600: 1524-1551.DOI: 10.1016/j.scitotenv.2017.04.238.
[17] Gu X G, Lu S G, Fu X R, et al.Carbon dioxide radical anion-based ![]() reductive process for carbon tetrachloride degradation in aqueous solution[J].Separation and Purification Technology, 2017, 172: 211-216.DOI: 10.1016/j.seppur.2016.08.019.
reductive process for carbon tetrachloride degradation in aqueous solution[J].Separation and Purification Technology, 2017, 172: 211-216.DOI: 10.1016/j.seppur.2016.08.019.
[18] Chen J L, Liu J Y, Zhou J S, et al.Reductive removal of nitrate by carbon dioxide radical with high product selectivity to form N2 in a UV/H2O2/HCOOH system[J].Journal of Water Process Engineering, 2020, 33:101097.DOI: 10.1016/j.jwpe.2019.101097.
[19] Harbour J R, Hair M L.Spin trapping of the·![]() radical in aqueous medium[J].Canadian Journal of Chemistry, 2011, 57(10): 1150-1152.DOI: 10.1139/v79-188.
radical in aqueous medium[J].Canadian Journal of Chemistry, 2011, 57(10): 1150-1152.DOI: 10.1139/v79-188.
[20] Cheng S A, Fung W K, Chan K Y, et al.Optimizing electron spin resonance detection of hydroxyl radical in water[J].Chemosphere, 2003, 52(10): 1797-1805.DOI: 10.1016/s0045-6535(03)00369-2.
[21] Han S K , Hwang T M, Yoon Y, et al.Evidence of singlet oxygen and hydroxyl radical formation in aqueous goethite suspension using spin-trapping electron paramagnetic resonance(EPR)[J].Chemosphere, 2011, 84(8): 1095-1101.DOI: 10.1016/j.chemosphere.2011.04.051.
[22] Augusto O,Bonini M G , Amanso A M, et al.Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology[J].Free Radical Biology and Medicine, 2002, 32(9): 841-859.DOI: 10.1016/S0891-5849(02)00786-4.
[23] Draganic Z D, Negronmendoza A, Sehested K, et al.Radiolysis of aqueous-solutions of ammonium bicarbonate over a large dose range[J].Radiation Physics and Chemistry, 1991, 38(3): 317-321.DOI:10.1016/1359-0197(91)90100-G.